相关热词搜索:
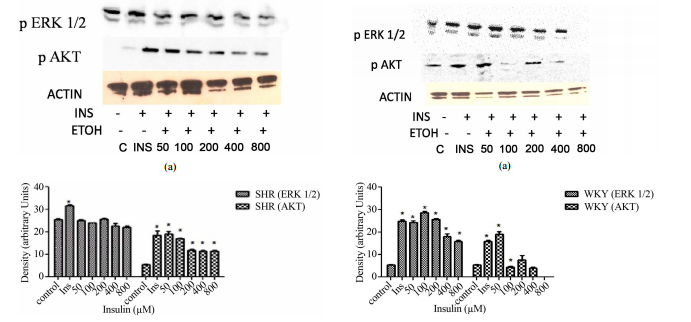
ABSTRACT Insulin resistance is an importantrisk factor in the development of cardiovascular diseases such as hypertensionand atherosclerosis. However, despite its importance, the specific role ofinsulin resistance in the etiology of these diseases is poorly understood. Atthe same time, ethanol (ETOH) is a potent vasoconstrictor that primarilyinduces down regulation of mitogen activated protein kinases (MAPKs) whichcould exacerbate insulin resistance and possibly lead to cardiovasculardiseases. This article describes how chronic ETOH exposure interferes withinsulin signaling in hypertensive vascular smooth muscle cells (HVSMCs) whichleads to the alteration of MAPKs, the major signaling molecules. Elevated (50 -800 mM) chronic exposure (24 hr) of HVSMCS to ETOH prior to insulin stimulationdecreased insulin-induced ERK 1/2 (MAPKs) and AKT expression. Similarexperiments were conducted using normotensive cells from rat. These cellsshowed reductions in insulin-induced ERK 1/2 phosphorylation as well, but onlyat higher concentrations of ETOH (400 - 800 mM). These alterations in insulinsignaling could provide an alternative molecular mechanism that may increasethe risk of insulin resistance, thus increasing the possibility ofcardiovascular diseases. Keywords: HVSMCs; Ethanol; Insulin; ERK 1/2; AKT 1.Introduction Insulin resistance can lead to the development of cardiovasculardiseases such as hypertension and atherosclerosis [1-3]. However, there islittle information known about the role of insulin resistance in cardiovasculardiseases. As a result of chronic alcohol consumption, insulin resistance canoccur. Insulin release and activation is important in the body to alleviate theoutcome of diabetes and in some cases, hypertension [4,5]. Chronic ethanol(ETOH) consumption can also affect insulin’s ability to bind to its receptor,therefore, leading to insulin resistance [4,5]. However, there are not manystudies to prove this effect, especially in a hypertensive phenotype. Studieshave shown that moderate consumption of alcohol is not a precursor to coronaryartery disease, but chronic consumption of alcohol can be detrimental [5].Insulin signaling is important in the cell for the release of insulin tocontrol glucose intake and lipid metabolism [5]. Insulin has an important rolein the biological process because it binds to its receptor (insulin receptor)to initiate glucose metabolism [5]. The insulin receptor is a transmembranedomain spanning tyrosine kinase receptor composed of alpha and beta subunitsthat mediate the actions of insulin [6-8]. Insulin is closely associated withhypertension, non-insulin dependent diabetes, atherosclerosis, and dyslipidemia[6,7]. As a result, insulin activation of PI3K-AKT (Phosphoinositide 3-Kinase),which mediates neuronal survival, motility, energy metabolism, and plasticityis impaired [6,8,9]. The extracellular signal-regulated kinases (ERKs), alsoknown as extracellular mitogen activated protein kinases (MAPKs), have beenfound to be altered by ethanol treatment of vascular smooth muscle cells from theaorta of a rat [5,9-11]. This effect of ETOH has been shown to be manifestedvia several pathways by the use of signaling inhibitors, such as PKC, leadingto a cascading effect in treated cells [11,12]. Several studies have shown thatinsulin activates a complex set of intracellular responses, including theactivation of mitogen- S. D. WILLIAMS, B. WASHINGTON 187 activated proteinkinases ERK 1/2 [13] and AKT [5]. The normal role of AKT in the cell is topropagate insulin receptor signaling to downstream effectors [14]. AKT isdownstream of PI3K, both of which are a part of one of the major pathways ininsulin signaling. The other major pathway is the mitogen activated proteinkinase pathway. It is also known that AKT has a role in activating insulin response[10]. Alteration of AKT expression is exhibited by ETOH impaired insulinsignaling in the body and a decrease in glucose transport of rat cardiac musclecells [10]. Although insulin response can be altered with chronic ETOH, thereare other factors that can alter this response such as genetics and theperson’s environment [5]. Chronic ETOH consumption in experimental animalmodels has been shown to alter insulin signaling events via the mitogenactivated protein kinases producing insulin resistance in the liver. Theseadverse effects of ethanol have been shown to be the result of the inhibitionof insulin or insulin-like growth factor which alters mRNA and DNA synthesisand the activation of proapoptotic signals through PI3K and AKT [15-17].Results from previous studies suggest that ETOH impairment of insulin action islikely to be downstream from PI3K, however, the mechanisms underlying theeffects of ETOH on insulin resistance and the effect of insulin resistance onthe development of cardiovascular diseases remain to be determined [5,18]. Thispaper describes how chronic ETOH exposure can alter insulin signaling in HVSMCsusing mitogen protein kinases as indicators. Chronic ETOH exposure’s effect oninsulin signaling has not been analyzed before in hypertensive cells. 2.Materials and Methods 2.1. Reagents and Antibodies ERK 1/2 (p44/p42) and AKTantibodies were purchased from Cell Signaling (Beverly, MA); anti-rabbit IgGantibodies (horseradish peroxidase linked) from Amersham Bioscience(Piscataway, NJ); and ECL detection system was obtained from PierceBiotechnology (Rockford, IL). Other supplies include Dulbecco’s ModifiedEagle’s Medium (Amersham), fetal bovine calf serum, penicillin and streptomycinwere purchased from Sigma/Aldrich (St. Louis, MO). 2.2. Cell Culture Vascularsmooth muscle cells (VSMCs) were received from Vanderbilt University. Cellswere cultured in DMEM containing 10% fetal bovine serum, 2% penicillin andstreptomycin. Subcultured passages were between 3 and 12. Cells were maintainedat a pH of 7.1 in 75 cm2 flasks under a humidified atmosphere of 5% CO2, 95% O2at 37˚C and plated in 6-well falcon plates. 2.3. Insulin Treatment For doseresponse experiments, cells were stimulated with 1, 2, 4, 8, and 16 μM insulinfor 30 min. Insulin was aspirated from each well. For time course experiments,cells were stimulated with 8 μM of insulin for 1, 5 10, 20, and 40 min. Cellswere lysed with 300 - 500 μl Laemmli Sample Buffer (2% SDS, 25% glycerol, 0.01%bromophenol blue, and 62.5 mM Tris-HCl pH 6.8). Cells were then scraped fromthe monolayer surface and collected in microcentrifuge tubes. 2.4. EthanolTreatment Hypertensive and normal rat cells were induced with 50, 100, 200,400, and 800 mM ETOH. Control cells were induced with a DMEM (-) solutioncontaining no serum that aids in cell proliferation. After a 24 hr incubationperiod, ETOH was aspirated from all wells and cells (except controls) werestimulated with 8 μM of insulin. Insulin was aspirated from each well and thecells were lysed with 300 - 500 μl Laemmli Sample Buffer (2% SDS, 25% glycerol,0.01% bromophenol blue, and 62.5 mM Tris-HCl pH 6.8). Cells were then scrapedfrom the monolayer surface and collected in microcentrifuge tubes. 2.5. WesternBlot Analysis Whole cell lysates were collected and diluted with sample bufferto equal concentrations of 40 μg/20 μl. Lowry protein assay was conducted todetermine standard protein concentration. Protein samples were then separatedalong with rainbow markers to measure the molecular weight of proteins on a 10%SDS-polyacrylamide gel from Bio-Rad Laboratories (Hercules, CA) at 200 voltsfor approximately 50 min. Proteins were then transferred to a nitrocellulosemembrane from Amersham Biosciences (Piscataway, NJ) using a semi-dry transferapparatus at 10 volts for 90 min. Blots were blocked with 2% non-fat dry milkin TBS (Tris-Buffered Saline) for at least one hour. After which blots wereincubated with primary antibodies ERK 1/2 or AKT overnight followed byanti-rabbit secondary antibody for 1 hour. Blots were immersed inchemiluminscent solution and developed in a dark room. 2.6. StatisticalAnalysis All experiments were performed in triplicate and expressed as means ±SE of the density using arbitrary units from three inpidual experiments.Statistical significance was determined with paired or unpaired onetailedStudent’s t-test, with P < 0.05 considered significant. Open Access CellBio188 S. D. WILLIAMS, B. WASHINGTON 3. Results 3.1. Insulin Induction IncreasesERK 1/2 and AKT Expression in Hypertensive VSMCs Before we could determine theeffect of ETOH on insulin signaling, we first had to determine the maximumconcentration of insulin it would take to stimulate phosphorylation of ERK 1/2and AKT via Western Blotting analysis. HVSMCs were stimulated with aconcentration range of 1 - 16 μM of insulin for 30 min. Figure 1 denotes thatstimulating HVSMCs with 1 - 16 μM of insulin, increased ERK 1/2 phosphorylationby approximately 23%. In addition, this insulin stimulation increased in AKTexpression 33% above basal with a maximum expression detected with 8 μM ofinsulin (Figure 2). This data suggests that AKT activation in HVSMCs is moresensitive than ERK 1/2 to insulin signaling. 3.2. Insulin Induction IncreasesERK 1/2 and AKT Expression in Normal VSMCs In order to determine the effect ofinsulin signaling in normal cells, VSMCs were stimulated with a concentrationrange of 1 - 16 μM of insulin. Maximal expression of phosphorylated ERK 1/2occurred with 8 μM of insu- (a) (b) Figure 1. Dose response curve for insulinon ERK 1/2 and AKT expression in HVSMCs. Cells were stimulated with 1, 2, 4, 8,and 16 μM insulin for 30 min and lysate harvested. (a) Western Blot Analysis ofSHR (Spontaneously Hypertensive) lysate probed with antibodies for ERK 1/2 andAKT expression; (b) Graphical representation of data by densitometry analysissoftware was taken from a mean of three experiments p < 0.05 compared tocontrol. (a) (b) Figure 2. Dose response curve for insulin on ERK 1/2 and AKTexpression in normal VSMCs. Cells were stimulated with 1, 2, 4, 8, and 16 μMinsulin for 30 min and lysate harvested. (a) Western Blot Analysis of WKY(Wistar Kyoto) lysate probed with antibodies for ERK 1/2 and AKT expression;(b) Graphical representation of data by densitometry analysis software wastaken from a mean of three experiments p < 0.05 compared to control. lin,which was significantly different from basal (Figure 2). No significantincreases in ERK 1/2 were observed between 1 - 4 mM (Figure 2). Similar experimentsshowed that AKT expression was significantly increased throughout allconcentrations except 1 μM with maximal expression at 8 μM. Insulin seemingly,also induced increases in AKT with 1 - 8 μM in normal VSMCs (Figure 2). Thisincrease in expression of AKT was not observed when cells were stimulated with16 μM insulin which resulted in complete inhibition. 3.3. Chronic ETOH ImpairsInsulin Signaling in Hypertensive VSMCs In order to investigate whether ETOHalters insulin signaling in HVSMCs, cells were treated chronically (24 hrs)with 50 - 800 mM of ETOH. After ETOH treatment HVSMCs were stimulated with 8 μMinsulin for 30 min and Western Blotting was performed. As a result, ERK 1/2expression significantly decreased with 50 - 800 mM ETOH treatment by 10%compared to insulin stimulation only (Figure 3). Using the same treatment range(50 - 800 mM) AKT expression was evaluated by chronic ETOH treatment. After 24hours ETOH treatment, AKT expression HVSMCs significantly decreased (Figure 3).This decrease was gradual as the concentration increased. Open Access CellBioS. D. WILLIAMS, B. WASHINGTON 189 (a) (b) Figure 3. Twenty-four hour ETOHtreatment of HVSMCs. Cells were treated with specified concentrations of ETOH(50 - 800 mM) and stimulated with 8 μM insulin for 30 min and lysate collected.(a) Western Blot Analysis of SHR (Spontaneously Hypertensive) lysate probedwith antibodies for ERK 1/2 and AKT expression; (b) Graphical representation ofdata by densitometry analysis software was taken from a mean of threeexperiments p < 0.05 compared to control. 3.4. Chronic ETOH Impairs InsulinSignaling in Normal VSMCs In order to investigate whether ETOH alters insulinsignaling in normal VSMCs, we evaluated chronic ETOH treatment in them as well.Cells were treated chronically with 50 - 800 mM of ETOH for 24 hr. After ETOHtreatment, VSMCs were stimulated with 8 μM insulin for 30 min. Fifty and 100 mMof ETOH had no significant effect on insulin-induced AKT expression whencompared to insulin stimulation alone. However, 200 - 800 mM reducedinsulin-induced AKT expression approximately 20%. Chronic ETOH treatment ofnormal VSMCs seems to cause an increase in signal at 50 mM and a decrease athigher concentrations when compared to the insulin induced increase alone(Figure 4). 4. Discussion The research findings in this paper provide evidencefor changes in mitogen-activated protein kinases possibly contributing to theonset of cardiovascular disease. The focus of this study is to show theassociation of chronic ethanol-induced changes in ERK 1/2 and AKT expression inHVSMCs. Recent reports show that hypertensive (a) (b) Figure 4. Twenty-fourhour ETOH treatment of normal VSMCs. Cells were treated with specifiedconcentrations of ETOH (50 - 800 mM) and stimulated with 8 μM insulin for 30min and lysate collected. (a) Western Blot Analysis of WKY (Wistar Kyoto)lysate probed with antibodies for ERK 1/2 and AKT expression; (b) Graphicalrepresentation of data by densitometry analysis software was taken from a meanof three experiments p < 0.05 compared to control. persons are predisposedto the development of diabetes. [19]. Seventy-five percent of cardiovasculardiseases are attributed to diabetes, hypertension, and alcohol, indicating thatthis is a contributing factor to the onset of hypertension. In Figure 1, ERK1/2 phosphorylation was increased in hypertensive cells with increasingconcentrations of insulin. This confirms that cells are able to survive withhigher concentrations of insulin indicated by ERK 1/2. On the other hand, whenAKT stimulation occurs, it has been reported that cellular expressiondecreases. This could be due to the alteration of insulin signaling. In Figure2, normal VSMCs with ERK 1/2 and AKT decreased with 1 - 8 μM insulin. With 16 μMof insulin, both ERK 1/2 and AKT expression was inhibited. In Figure 3,hypertensive cells treated with ethanol ERK 1/2 and AKT both decrease inexpression as the concentrations of ETOH increase which could mean that ethanolis affecting insulin signaling. Figure 4 depicts a biphasic effect of ETOH oninsulin signaling in normal cells which suggest that there may be differenteffect in normal cells. Cardiovascular diseases include atherosclerosis andhypertension. Risk factors for such diseases of the cardiovascular systeminclude family history, diabetes, obeOpen Access CellBio 190 S. D. WILLIAMS, B.WASHINGTON sity, smoking, excessive alcohol intake, and a diet high in saltand/or low in antioxidant nutrients. Inpiduals with hypertension are atincreased risk for atherosclerotic diseases such as stroke, heart, and kidneydisease which can be exacerbated by diabetes and alcohol [20]. The adverseeffects of long-term excessive use of alcohol are similar to those seen withother sedative-hypnotics drugs (apart from organ toxicity which is much moreproblematic with alcohol). Though the underlying mechanisms remain undefined,accumulating evidence strongly suggests that ETOH interferes with insulin’saction by altering mitogen activated protein kinases. The major signalingmolecules, MAPKs, implicated in the biological actions of insulin, and theexpression of the insulin receptor may be major factors leading tocardiovascular diseases. 5. Acknowledgements The authors would like toacknowledge The Research and Engineering Training Program (REAP) and Title IIIProgram for providing support for this work.