相关热词搜索:
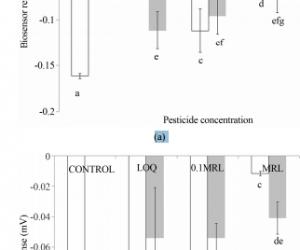
ABSTRACT Organophosphates belong to themost important pesticides used in agricultural practice worldwide. Althoughtheir analytical determinations are quite feasible with various conventionalmethods, there is a lack of efficient screening methods, which will facilitatethe rapid, high-throughput detection of organophosphates in different foodcommodities. This study presents the construction of a rapid and sensitivecellular biosensor test based on the measurement of changes of the cellmembrane potential of immobilized cells, according to the working principle ofthe Bioelectric Recognition Assay (BERA). Two different cell types were used,derived either by animal (neuroblastoma) or plant cells (tobacco protoplasts).The sensor was applied for the detection of a mixture of two organophosphatepesticides, diazinon and chlorpyrifos in two different substrates (tomato,orange). The pesticides in the samples inhibited the activity of cellmembrane-bound acetylcholinesterase (AChE), thus causing a measurable membranedepolarization in the presence of achetylcholine (Ach). Based on the observedpatterns of response, we demonstrate that the sensor can be used for thequalitative and, in some concentrations, quantitative detection oforganophosphates in different substrates with satisfactory reproducibility andsensitivity, with a limit of detection at least equal to the official Limit ofDetection (LOQ). The assay is rapid with a total duration of 3 min at acompetitive cost. The sensitivity of the biosensor can be further increasedeither by incorporating more AChE-bearing cells per test reaction unit or byusing cells engineered with more potent AChE isoforms. Standardization ofcultured cell parameters, such as age of the cells and subculture history priorto cell immobilization, combined with use of planar electrodes, can furtherincrease the reproducibility of the novel test. Keywords: BioelectricRecognition Assay (BERA); Matrix Effects; N2a Cells; Organophosphates; TobaccoProtoplasts 1. Introduction Organophosphate insecticides have been used widelyin agriculture and in household applications as pesticides due to their highinsecticidal activity and relatively low persistence [1]. Their mechanism ofaction is the irreversible inhibition of acetylcholinesterase (AchE), a keyenzyme in the recycling of the neurotransmitter acetylcholine (Ach) [2].Organophosphates phosphorylate the serine hydroxyl group at the site of actionof acetylcholine. They bind irreversibly, deactivating the esterase, resultingin accumulation of acetylcholine at the endplate. Decrease in plasmacholinesterase results in a decrease of cholinesterase activity in the central,parasympathetic, and sympathetic nervous systems. Accumulation of acetylcholineat the neuromuscular junction causes persistent depolarization of skeletalmuscle, while neural transmission in the central nervous system is disrupted[3]. Long-term exposure to organophosphates has been associated withirritability, fatigue, headache, difficulties with memory and concentration andother neurophysiological abnormalities [4-6]. The conventional analysis ofpesticide residues in food commodities is a labor intensive procedure, since itis necessary to cover a wide range of different chemicals, using a singleprocedure. Standard analysis methods include gas chromatography and highperformance liquid chromatography to achieve the necessary selectivity andsensitivity for the different classes of compounds under detection [7]. As aconsequence, current methods of analysis provide a limited sample analysiscapacity, on a * day/instrument basis [8]. While the analytical determina-Corresponding author. Copyright © 2013 SciRes. CellBio 132 K. LOKKA ET AL. tionof the pesticide residues in an unknown sample will always be carried out usingsophisticated methods based on conventional technology, rapid screening is theonly solution for assuring food control by means of highthroughput detection oforganophosphates in different food commodities. Providing novel solutions forfood quality monitoring is also in accordance with the new EU and internationalregulations for minimal residue concentration in marketed food and agriculturalproducts. Based on the inhibition of AchE and choline oxidase, many biosensorshave been developed for the detection of organophosphorous and carbamatepesticides such as those described by Andres et al. [9], Choi et al. [10] andAndreou et al. [11]. It has been recently demonstrated that a successfulpesticide assay could be based on a BERA platform, comprising electricallyactive cells (i.e. with an active transport of ions through the cell membrane)interfaced with microelectrodes which allow the capture of extracellular spikesor impedance changes associated with cellular response against the pesticideunder detection [12]. More specifically, the inhibition of AchE byorganophosphates and carbamate residues caused an increase of available Ach inthe assay solution, which in turn caused the depolarization of the membranes ofimmobilized neuronal cells in a concentrationdependnet manner. In other words,the presence of the pesticides was detected by the degree of inhibition ofcellular AChE, which is inversely associated with ACh concentration. Inhibitionof AChE leads in increased excitatory ACh transmission and depolarization ofthe cell membrane, which was measured as a change of the sensor’s potential,due to changes in the concentration of electrolytes in the immediate vicinityof the working electrode. This novel type of biosensor was further included ina validation test against E.U. proficiency test samples [13]. The aim of thepresent study was to further develop this novel biosensor principle byincorporating, for the first time, plant (tobacco) protoplasts asorganophosphate biorecognition elements. Their reliability as sensor componentswas compared to those of neuronal cells. In addition, both biosensor versionswere applied for the detection of a mixture of organophosphate pesticides indifferent substrates, thus evaluating, also for the first time, possible matrixeffects on the sensor’s performance. 2. Materials and Methods 2.1. MaterialsDiazinon (Diethoxy-[(2-isopropyl-6-methyl-4-pyramidinyl)oxy]-thioxophosphorane;CAS [333-41-5]; Mw = 304.35) and chlorpyrifos (O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate; CAS [2921-88-2]; Mw = 350.59) (both purchased fromChem Device, West Chester, PA, USA) were used as standard organophosphateinsecticides. Pesticide mixtures which contained 10 μM of each pesticide, wereprepared daily in acetone solution. All other reagents were purchased fromFluka (Switzerland). 2.2. Cell Culture and Sensor Fabrication from PlantProtoplast and Neuroblastoma N2a Cells Plant protoplasts were isolated fromtobacco (Nicotiana tabacum) leaves by preplasmolysing 0.5 g of them in 20 ml ofCPW solution [14] supplemented with 0.7 M mannitol for one hour and thenincubating them in 20 ml solution of the same composition and additionallysupplemented with 3 mg pectinase (8.5 units/mg, from Aspergilus niger) and 2 mgcellulase (9.5 units/mg, from Trichoderma viridae) for 20 hours. One ml ofprotoplast solution contained 3.5 × 106 cells/ml. Mouse neuroblastoma (N2a)cell cultures were originally provided from LGC promochem (UK). Cells werecultured in Dulbecco’s medium with 10% heat-inactivated foetal calf serum(FCS), 10% antibiotics (streptomycin) and 10% l-glutamine. For incorporationinto the biosensor, cells were detached from the culture vessel by addingtrypsine/ EDTA for 10 min at 37˚C and further concentrated by centrifugation (6min, 1200 rpm, 25˚C). For manufacturing consumable biosensors, 1 ml of plant oranimal cells (at a density of 2.5 × 106 /ml) were mixed with 2 ml of 4% (w/v)sodium alginate solution and then the mixture was added drop wise, by means ofa 22G syringe, in 0.8 M CaCl2. Each of the resulting calcium alginate beads hadan approximate diameter of 2 mm and contained approximately 5 × 104 cells. Thesensors were storable at room temperature in culture medium, under normalatmospheric conditions (i.e. non-CO2 enriched) for at least three weeks withoutany loss of performance. 2.3. Sample Preparation Organically-grown,pesticide-free tomato and orange fruits were used for the preparation of theassayed samples. Two groups of samples were prepared: The first group(fortified samples) comprised of blended fruits spiked with mixtures ofdiazinon and chlorpyrifos at various concentrations. Subsequently, 15 g of theblended fruit-pesticide mixture were homogenized in 30 ml acetone using anUltra-Turrex homogeniser (model Silent Crusher, Heidolph) at 6000 rpm for onemin. In following, 30 ml dichloromethane, then 30 ml petroleum ether were addedand the mixture was stirred for one min. Then the mixture was centrifuged at4000 rpm for 4 min at 20˚C. After removing 25 ml of the supernatant, the restwas left to dry in a waterbath, at 65˚C - 70˚C. Finally, the dry samples wereredissolved in 5 ml methanol (10% v/v). The second group (matrix standards) wascreated Copyright © 2013 SciRes. CellBio K. LOKKA ET AL. 133 by spiking finaldry samples, dissolved in methanol, with the pesticide mixtures. Theconcentration of each pesticide in the samples was calculated so that eachpesticide was present either at the Limit of Quantification (LOQ), the MinimumResidue Level (MRL) or one-tenth thereof (MRL/10), according to theconcentrations specified by the European Union Directive 839/2008 (Table 1).Control samples were created as the analytical sample but omitting spiking withpesticide mixtures. Dried control samples were also redissolved in methanol. 2.4.Assay Principle According to the working principle of the method, the presenceof organophosphate compounds is detected by the degree of inhibition ofcellular AChE, which is inversely associated with ACh concentration. ACh is anexcitatory neurotransmitter (Hoang et al., 2007), the activity of which isregulated by AChE. Therefore, inhibition of AChE can lead in increasedexcitatory ACh transmission, which can be measured by the depolarization of thecell membrane. In other words, inhibition of AChE by pesticide residues in thesample will result to excessive stimulation of N2a cells by ACh, which willfurther lead to membrane depolarization above a predetermined threshold.Membrane depolarization events and associated electrolyte influx/efflux will reflectthemselves on the sensor’s response as a change of the sensor’s potential, dueto changes in the concentration of electrolytes in the immediate vicinity ofthe working electrode [12,13]. 2.5. Assay Procedure Each cell-bearing bead(cell sensor) was manually connected to a working electrode (the electrode wasinserted through the entire length of the sensor without extruding from theopposite end) made from pure silver, electrochemically coated with an Ag/AgCllayer and having a diameter of 0.75 mm. The distance between working andreference electrode was 3 cm. Electrodes were connected to the recordingdevice, which comprised the PMD-1608FS A/D card (Measurement Computing,Middleboro, MA) (Figures 1(a) and (b)). The software responsible for recordingthe signal and data processing was InstaCal (Measurement Computing). For eachassay, the sensor system, comprising of the Table 1. LOQ and MRL points laiddown by the European Union Directive 839/2008. Tomato Orange LOQ (ppm) MRL(ppm) LOQ (ppm) MRL (ppm) Diazinon 0.02 0.5 0.024 1 Chlorpyrifos 0.03 0.5 0.030.3 bead attached to the working electrode and a reference electrode, wasimmersed into each sample solution (200 μl). The sample solution comprised 150μl of 50 mM Tris-aminomethane (Tris) buffer (pH 8), containing 0.5 mMacetylcholine iodide (Ach) and 50 μl of pesticide sample. The response of eachsensor was estimated by recording the average change of the sensor potentialfor a period of 180 sec after sample application. 2.6. Data Analysis andExperimental Design Experiments were set up in a completely randomized designand each experiment was repeated three times. In each application, a set of sixbiosensors was tested against each inpidual sample. Correlations between thesensor’s response and pesticide concentrations were done using MS-Excel. Datameans among different days were compared using Duncan’s multiple range test(with significance at p < 0.05). The effect of the extraction procedure andthe concentration of the pesticides on the screening efficiency (SE%) in eachsample was calculated inpidually for each sample, according to the followingequation: average response to the fortified sample % 100 average response tothe matrix standard ′ =SE 3. Results and Discussion3.1. Response of the Sensor to Organophosphate Pesticides in Tomato Samples Theresponse of the cell-based biosensor against different concentrations of amixture of diazinon and chlorpyrifos in tomato samples is presented in Figure2. The results Figure 1. (A) Schematic outline of the biosensor system. Thegraph shows the considerable increase of the response of the cellularbiorecognition element to Ach after the addition of an organophosphate(chlorpyriphos) (red line). The insert (B) shows a Petri dish with consumablebiorecognition elements (gel beads with immobilized cells). Copyright © 2013SciRes. CellBio 134 K. LOKKA ET AL. of the assay using neuroblastoma as thesensor’s biorecognition elements are shown in Figure 2(a), while the results ofthe assay using plant protoplasts are shown in Figure 1(b). In the case ofneuroblastoma N2a cells, when no pesticide was present in the sample (control(a) (b) Figure 2. Response of the cell-based biosensor against differentconcentrations of a mixture of diazinon and chlorpyrifos in tomato samples. Thebiosensor was based either on neuroblastoma cells (a) or tobacco protoplasts(b). Pesticide concentrations are expressed as the corresponding LOQ, MRL andMRL/10 values, according to Table 1. Sensor response is expressed as a changein the membrane potential of immobilized cells. (n = 6 replications (differentsensors) for each sample and error bars represent standard errors of theaverage value of all replications with each sample). The white columnsrepresent matrix standards and the grey columns fortified samples, as describedanalytically in the Materials section. Columns sharing a common letter are notstatistically different (p ≥ 0.05). sample), a sensor response of −142 ± 2 mVwas observed. The sensors responded to increasing pesticide concentrations byconsiderable positive increase of the sensor’s potential (Figure 2(a)). Thisobservation is in accordance with the assay principle, where inhibition of AChEcan lead in increased excitatory ACh transmission, which can be measured by thedepolarization of the cell membrane (hence the shift of the sensor measurementsto more positive values). The response of the sensor against matrix standardswas quite reproducible (average variation = 12.7%) with the exception of theresponse against the highest concentration (MRL). Considering matrix standardsonly, a satisfactory correlation was observed between sensor response and totalpesticide concentration (r2 = 0.9624, y = 0.042x – 0.1765). On the contrary,the response against the fortified samples was less reproducible, although aconcentration-dependent pattern was again observed. The better reproducibilityof the response of matrix standards vs. fortified samples could be due to themore homogenous distribution of the pesticides under detection in the matrixstandards compared to the fortified samples. When plant protoplasts were used,the response to control solutions was less negative (−95 ± 1 mV) than withanimal cells, indicating a lower state of membrane hyperpolarization of theimmobilized cells. In this case, a correlation between pesticide concentrationand biosensor response was observed only against matrix standards, notfortified samples (Figure 2(b)). In addition, a considerable variation of thesensor response was observed, much higher than for the N2a-based sensor.Depending on the concentration of the pesticides, the screening efficiencyranged from 42% to 141%. Thus, spiked pesticide concentrations lower than orequal to the LOQ were underestimated, while higher concentrations (0.1 MRL)were overestimated. 3.2. Response of the Sensor to Organophosphate Pesticidesin Orange Samples The response of the cell-based biosensor against differentconcentrations of a mixture of diazinon and chlorpyrifos in orange samples ispresented in Figure 3. The results of the assay using neuroblastoma as thesensor’s biorecognition elements are shown in Figure 3(a), while the results ofthe assay using plant protoplasts are shown in Figure 3(b). When no pesticidewas present in the sample (control sample), a sensor response of −161 ± 3 mV(animal cells) or −119 ± 7 mV (plant protoplasts) was observed, i.e.steady-state membrane hyperpolarization was higher than for tomato controls.This was probably due to the different matrix effect: Mizayawa et al. [15] havepreviously reported that constituents of the essential oils of Citrus sp.considerably affected the activity of Copyright © 2013 SciRes. CellBio K. LOKKAET AL. 135 (a) (b) Figure 3. Response of the cell-based biosensor againstdifferent concentrations of a mixture of diazinon and chlorpyrifos in orangesamples. The biosensor was based either on neuroblastoma cells (a) or tobaccoprotoplasts (b). Pesticide concentrations are expressed as the correspondingLOQ, MRL and MRL/10 values, according to Table 1. Sensor response is expressedas a change in the membrane potential of immobilized cells. (n = 6 replications(different sensors) for each sample and error bars represent standard errors ofthe average value of all replications with each sample). The white columnsrepresent matrix standards and the grey columns fortified samples, as describedanalytically in the Materials section. Columns sharing a common letter are notstatistically different (p ≥ 0.05). membrane-bound AChE, thus affecting thepotential difference along the membrane of the immobilized cells. Similarly totomato samples, the response of the sensor dependent on the total pesticideconcentration, although this effect was less pronounced using the plantcell-based version (Figure 3(b)). In addition, the response against orangesamples was more reproducible than for tomato samples, especially againstanalytical orange samples. This lack of reproducibility (i.e. a considerablevariation of response) was even more obvious when protoplastbased sensors wereused to assay fortified samples, especially at the LOQ (Figure 3(b)). Dependingon the concentration of the pesticides, the screening efficiency ranged from38% to 350%, i.e. pesticide concentrations were either under- or overestimated,depending on the spiked concentration and the type of the biosensor, similarlyto the assay of the tomato samples. On the other hand, and considering onlymatrix standards, a satisfactory correlation was observed between sensorresponse and total pesticide concentration for both animal cell-based (r2 =0,8947, y = 0.0018x – 0,1454) and plant protoplast-based biosensors (r2 =0.7923, y = 0.0321x – 0.1593). However, as indicated by theconcentration-dependent screening efficiency for both types of biosensors, it wasnot possible to obtain a reliable quantitative response. By testing morepesticide concentrations in the future, it might be possible to identify arange of concentrations for each matrix X biosensor combination where thescreening efficiency will be close to 100%, thus allowing for quantitativedetermination as well. Differences in the sensor responses between tomato andorange samples pesticides may be due to differences of the matrix effect. Forexample, tomato is rich in carotenoids, such as lycopene. It has beenpreviously shown that carotenoids and their oxidation products promote gapjunctional communication [16], therefore possibly affecting theelectrophysiological behaviour of neuroblastoma cells. Matrix effects aside,differences in biosensor response to different batches of the same sample couldbe due to factors related to the cellular biorecognition element itself, suchas the age of the cells (days elapsed between the detachment of cells fromculture, subsequent immobilization and use of the biosensor) as well assubculture history prior to cell immobilization. It is strongly recommended tostandardize these factors in order to minimize variability in biosensorresponse. As expected, biosensors based on neuroblastoma cells were moresensitive than protoplast-based ones, as demonstrated by the greater differencein response (cell membrane depolarization) compared to control samples. This isdue to their membrane-bound AChE [17]. The occurrence of this enzyme on themembranes of tobacco protoplasts is also a fact, as first reported by Madhavanet al. [18] (though in guard, not mesophyll cells). However, the concentrationof the enzyme on the plant cell membrane is considerably lower than onneuroblastoma cells, as corroborated by colorimetric assays by our group(unpublished data). Yet the sensitivity of the sensor sysCopyright © 2013SciRes. CellBio 136 K. LOKKA ET AL. tem presented in this study is high enoughto allow for the measurement of the pesticide-AChE interaction, even at theLOQ. The AChE-based higher level of response of neuroblastoma cells could befurther exploited in order to increase the sensitivity of the novel assay,either by incorporating more AChE-bearing cells per test reaction unit, or byusing cells engineered with more potent AChE isoforms. The first approach iscurrently under investigation in our laboratory with promising, thoughpreliminary, results. The reproducibility of the system could be improvedconsiderably by redesigning the cell-electrode interface. Quite recentexperiments in our lab has shown that using screen-printed electrodes reducedvariation in response against organophosphate pesticides in solution, though nomatrix effects were yet assessed [19]. We plan to repeat the experimentsdescribed in the present study using planar electrodes. The novel assay hasalso been used for the detection of the avermectin abamectin and the pyrethroidα-cypermethrin [20], whereas various operational parameters, including assaytemperature and electrode material were validated. A variation of theexperimental approach described in the present study involved the seeding ofN2a neuroblastoma cells on top of PEDOT electrodes treated with Nafion andPolylysine [21]. 4. Conclusion There are several areas of future work that wouldimprove the utility of cell-based biosensors. Numerous applications wouldbenefit from the development of parallel systems that allow for simultaneousmeasurements on multiple cell lines, thus improving both the breadth ofsensitivity and the ability to discriminate or classify different groups ofanalytes. As demonstrated in the present study, the biosensor system can beused only for screening purposes, since it was not possible to achievesatisfactory quantitative determination. In addition, due to the vastdifferentiation of food commodities, a much wider range of samples, both in thecontext of matrix and residue composition should be tested before the novelsystem can be practically employed. Despite this, the novel biosensor forscreening organophosphate pesticides offers a number of advantages over otherconventional biosensor techniques, such as sensitivity and low cost, as well asthe ability to monitor, in real-time, the presence of pesticide residues infood products. A particular advantage is the high speed of the assay (analysistime = 3 min), although this does not include the time required for sampleextraction. However, we should mention that, for the practical application ofthe novel assay as a rapid screening tool, a single-or two-step extraction ofthe sample in an organic solvent would suffice. It must be emphasized that itis not meant to replace elaborate analytical methods but rather to assist thescaling up of food quality control, primarily designed to screen rapidly largeamounts of agricultural products and food commodities for the presence ofpesticide residues at the site of production (field), packaging, processingand/or sale. The application scope of the novel assay principle has alreadybeen extended to include complex organic contaminants [22] and mycotoxins [23].Therefore, it represents a totally new generation of analytical instruments,enabling the implementation of food safety analysis by even minor users, suchas small agricultural unions or food companies and can be potentially used byall parties involved in the chain of food production, processing anddistribution, a market with a volume of 3.6 billion
 2
2


