相关热词搜索: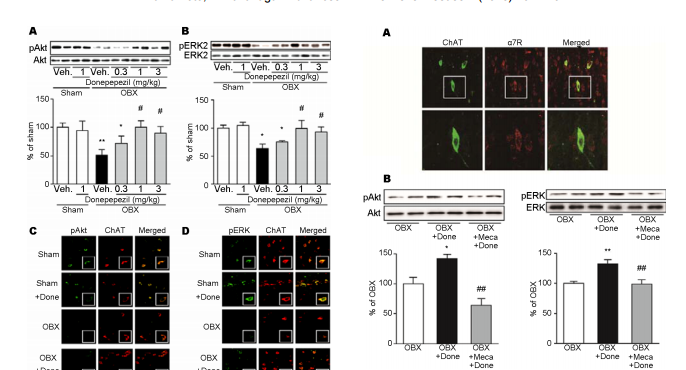 Donepezil rescues the medical septum cholinergic neurons via nicotinic ACh receptor stimulation in olfactory bulbectomized mice Yui Yamamoto, Kohji Fukunaga* Department of Pharmacology, Graduate School of Pharmaceutical Science, Tohoku University, Sendai, Japan; * Corresponding Author: [email protected] Received 12 September 2013; revised 20 October 2013; accepted 1 November 2013 Copyright © 2013 Yui Yamamoto, Kohji Fukunaga. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Olfactory bulbectomy (OBX) causes cognitive dysfunction by degeneration of cholinergic neurons in the medial septum. Here, we define an involvement of nicotinic acetylcholine receptor (nAChR) in neuroprotective effect of donepezil in the septum neurons of OBX mice. Neuroprotective effects on the medial septal cholinergic neurons were assessed after chronic donepezil administration in OBX mice. We also measured Akt and ERK phosphorylation to define the neuroprotective mechanism of donepezil. We found that treatment with donepezil (1 - 3 mg/kg) for 15 consecutive days completely rescued cholinergic neurons in the OBX mice with concomitant improved memory. Reduction of both protein kinase B (Akt) and extracellular signal-regulated kinase (ERK) phosphorylation were restored by chronic donepezil administration (1 - 3 mg/kg) in OBX mouse medial septum. Both phosphorylated Akt and ERK immunoreactivities were localized in cell bodies of choline acetyltransferase (ChAT)-positive cholinergic cells in the medial septum. Enhancement of Akt and ERK phosphorylation seen following donepezil administration was totally blocked by pre-administration of mecamylamine (10 μM), a nicotinic acetylcholine receptor antagonist. Donepezil increases phosphorylation of Akt and ERK via nAChR stimulation in the medial septum cholinergic neurons. The Akt and ERK stimulation by donepezil is associated with its ability of neuroprotection in the medial septum and memory improvement. Keywords: Donepezil; Neuroprotection; Medial Septum; Nicotinic Acetylcholine Receptors 1. INTRODUCTION Destruction of olfactory system reportedly impairs learning and memory [1-3] and Alzheimer’s disease (AD) patients show severe impairment of olfactory bulb functions even in the early stage [4,5]. The cholinergic neurons in the basal forebrain and olfactory bulb are originated from the medial septum, thereby providing cholinergic innervation to the hippocampus, olfactory blub, amygdala and overall neocortex [6]. Interestingly, olfactory bulbectomy (OBX) causes degeneration of cholinergic neurons not only in the medial septum but also in the hippocampus, thereby eliciting hippocampus -dependent memory deficits in mice [7]. In support to this idea, OBX treatment elicits reduction of acetylcholinesterase (AChE) and choline acetyltransferase (ChAT) levels in the hippocampus [8,9] and nicotine-induced acetylcholine (ACh) release in the hippocampus was largely impaired in OBX mice [10]. In addition, significant elevation of betaamyloid (Aβ) levels is seen in extracts from neocortex and hippocampus by OBX in mice [11]. Thus, OBX mouse could be a useful model to test AD-like cognitive dysfunction following degeneration of cholinergic neurons in the septum. Donepezil, an AChE inhibitor, is prototyped therapeutics for Alzheimer’s disease (AD) patients in the worldwide [12]. Donepezil elevates extracellular ACh levels in the cortex [13,14]. Interestingly, neuroprotective effects of donepezil were also found both in vitro and in vivo. For example, donepezil blocked glutamate-induced excitotoxicity in rat primary cultured neurons and oxygen-glucose deprivation-induced cell death in HEK293 Copyright © 2013 SciRes. OPEN ACCESS 162 Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 cells and cortical neurons [15-18]. Donepezil also attenuates streptozotocin-induced or traumatic brain injury-induced neuronal death in hippocampal CA1 region, thereby improving cognitive impairment [19,20]. In human AD patients, donepezil slowed inhibition of hippocampal atrophy [21]. However, the mechanism underlying donepezil-mediated neuroprotection remains unclear. Donepezil treatment activated phosphatidylinositol 3- kinase (PI3K)/protein kinase B (Akt) and increased expression of Bcl-2, an anti-apoptotic protein, in rat cortical neurons [22]. Donepezil also potentiates the nerve growth factor (NGF)-induced phosphorylation of extracellular signal-regulated kinase (ERK) in PC12 cells [23]. Furthermore, neuroprotective effects of donepezil were abolished by mecamylamine, the non-selective nicotinic acetylcholine receptors (nAChRs) inhibitor, but not with scopolamine, the muscarinic AChRs (mAChRs) inhibitor, in rat cortical neurons [22]. Increased Akt phosphorylation following donepezil treatment in Aβ42-exposed cortical cultured neurons was blocked by mecamylamine pre-treatment [24]. Nicotine-induced activation of ERK was also blocked by mecamylamine in cultured spinal cord neurons [25]. Here, we found that chronic donepezil administration rescue degeneration of medial septum cholinergic neurons observed in OBX mice. Furthermore, we defined the expression of nAChR in the medial septum cholinergic neurons and possible neuroprotective mechanisms of donepezil in OBX mouse medial septum. 2. MATERIALS AND METHODS 2.1. Animals Adult male DDY mice 6 weeks old (Nippon SLC, Hamamatsu, Japan) housed in cages with free access to food and water at a constant temperature (23˚C ± 1˚C) and humidity (55% ± 5%) with a 12-h light/dark cycle (09:00 - 21:00 hours). All experimental procedures using animals were approved by the Committee on Animal Experiments at Tohoku University Graduate School of Pharmaceutical Sciences. 2.2. Establishment of Bilateral Olfactory Bulbectomy Mice and Drug Treatment OBX mice were prepared as described [9,10,26]. Briefly, mice anesthetized with sodium pentobarbital (50 mg/kg, i.p., Dainippon, Osaka, Japan) were placed in a stereotaxic instrument. After exposure of the skull, 1-mm diameter holes were drilled on either side of the olfactory bulbs, which were then removed by gentle aspiration by a suction pump. Care was taken not to damage the frontal cortex. Holes were filled with a hemostatic sponge to avoid bleeding and the skin was closed. Sham-operated mice were treated similarly but bulbs were left intact. Drug administrations were begun 3 days after recovery period. Donepezil (0.3, 1 or 3 mg/kg, dissolved in distilled water or vehicle alone) was administered orally daily for 15 consecutive days until mice were sacrificed, or one time intraperitoneally 30 min before sacrificed at 18 days after OBX surgery. Mecamylamine (10 μM) or saline were injected into the left brain lateral cerebroventricle 60min before acute donepezil administration. Following sacrificed by decapitation, medial septum was dissected out for further analyses. 2.3. Behavioral Analyses Spontaneous alternation behavior in a Y-maze served as a spatial reference memory task. The maze was made of black acryl plate. Each arm was 40 cm long, 8 cm high, 3 cm wide at the bottom and all three arms converged at an equal angle. Each mouse was placed at the end of a fixed arm and allowed to move freely through the maze during an 8-min session. The sequence of arm entries was recorded manually. An alternation was defined as entries into all three arms on consecutive choices. The maximum number of alternations was defined as the total number of arms entered minus two, and the percentage of alternation was calculated as (actual alternations/maximum alternations) × 100. The total number of arms entered during the session was also determined. The novel object recognition task used to evaluate recognition memory [27]. This task is based on the tendency of rodents to discriminate a familiar from a new object. Mice were inpidually habituated to an openfield box (35 × 25 × 35 cm) for 2 consecutive days. The experimenter scoring behaviors were blinded to the treatment. During the acquisition phases, two objects of the same material were placed symmetrically in the center of the chamber for 10 min. One hour after acquisition phase training, one object was replaced by novel object, and exploratory behavior was again analyzed for 5 min. After each session, objects were thoroughly cleaned with 75% ethanol to prevent odor recognition. Exploration of an object was defined as rearing on the object or sniffing it at a distance of less than 1 cm, touching it with the nose, or both. Successful recognition of a previously explored object was reflected by preferential exploration of the novel object. Discrimination of spatial novelty was assessed by comparing the difference between time of exploration of the novel (right) and familiar object (left) and the total time spent exploring both objects. 2.4. Immunohistochemical Studies Sham and OBX mice were treated with vehicle or donepezil (1 mg/kg) from 3 days to 17 days after surgery. Copyright © 2013 SciRes. OPEN ACCESS Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 163 At 18 days after surgery, mice anesthetized and perfusion-fixed with 4% paraformaldehyde. Briefly, after fixation, 50 μm thick coronal sections were cut using a vibrating microtome (Dosaka EM Co. Ltd., Kyoto, Japan). Sections were incubated as follows: for 30 min in 0.1 M PBS (pH 7.4) containing 0.1% Triton X-100; for 1 h in PBS with 3% BSA (blocking solution); overnight with various primary antibodies in blocking solution at 4˚C. For ChAT-immunostaining, sections were incubated with goat anti-ChAT polyclonal antibody (1:1000) (Millipore Bioscience Research Reagents, Temecula, CA). After washing, sections were incubated with biotinylated anti-goat IgG (1:5000) (Jackson Immuno Research Laboratories, West Grove, PA, USA) in TNB buffer for 1 h, followed by streptavidin-horseradish peroxidase (1:5000) (NEN Life Science Products, Boston, MA) labeled for 2 h. Sections were then stained with tetramethylrhodamine tyramide for 10 min using the TSA-Direct kit (NEN Life Science Products). For double staining with ChAT and phosphorylated Akt or ERK, sections were incubated with goat anti-ChAT polyclonal antibody (1:1000) (Millipore), a rabbit anti-phosphorylated Akt (Thr-308) polyclonal antibody (1:1000) (Millipore) or a rabbit antiphosphorylated ERK (Thr-202/Tyr-204) polyclonal antibody (1:1000) (Cell Signaling Technology, Beverly, MA, USA). After thorough washing, sections were incubated 3 h with Alexa 594-labeled anti-goat IgG or Alexa 488-labeled anti-rabbit IgG. To detect α7 nAChR, sections were incubated in α-bungarotoxin, tetramethylrhodamine conjugate (α-BTX) (Molecular Probes, Eugene, Oregon, USA) for 2 h at 37˚C, followed by incubation in antibodies for ChAT, phospho-Akt or phospho-ERK overnight at 4˚C. After several washes in PBS, sections were mounted on slides with Vectashield (Vector Laboratories, Burlingame, CA). Immunofluorescent images were analyzed using a confocal laser scanning microscope (Leica TCS, Olympus, Tokyo, Japan). 2.5. Immunoblotting Analysis In Figures 1 and 2, sham and OBX mice were treated with vehicle or donepezil (1 mg/kg) from 3 day to 17 days after OBX. And at 18 days, Medial septum was dissected out and stocked until homogenization. In Figure 3, sham and OBX mice were administrated donepezil (1 mg/kg) and mechamylamine as indicated. One hr after administration, the septum is dissected out from each mouse. The septum samples were homogenized in 70 µl of buffer containing 50 mM Tris-HCl, pH 7.4, 0.5% Triton X-100, 4 mM EGTA, 10 mM EDTA, 1 mM Na3VO4, 40 mM sodium pyrophosphate, 50 mM NaF, 100 nM calyculin A, 50 µg/ml leupeptin, 25 µg/ml pepstatin A, 50 µg/ml trypsin inhibitor, and 1 mM dithiothreitol. Insoluble material was removed by a 10 min centrifugation (15,000 rpm). Samples containing equivaFigure 1. Effects of donepezil administration on cholinergic neurodegeneration in OBX mouse medial septum. (A) Representative brain sections showing changes in numbers of cholinergic neurons. (a), cholinergic neurons were identified by ChAT immunostaining in sham (b), donepezil treated (1 mg/kg daily for 15 days) sham mice (c), vehicle (d), or donepezil treated (1, 3 mg/kg daily for 15 days) (e), (f) OBX mice. Confocal laser scanning images showed predominant ChAT expression in cell bodies of cholinergic neurons in the medial septum indicating in box (a). Scale bar, 150 μm. (B) Quantitative analysis of ChAT levels was undertaken by counting ChAT-positive neurons. Data are expressed as percentage of values of sham-operated animals (mean ± S.E.M.) (n = 6). * P < 0.05 versus sham-operated animals and # P < 0.05 versus vehicle-treated mice. Dunnett’s multiple comparison tests was used for data analysis. (C) ChAT levels were detected by immunoblotting with or without treatment with donepezil. Quantitative analysis of 68 KDa ChAT levels were performed by densitometric analysis of immunoblots. Immunoblots with β- tubulin showed equal protein loading. Data are expressed as percentage of values of sham-operated animals (mean ± S.E.M.) (n = 6). **P < 0.01 versus sham-operated animals and # P < 0.05 versus vehicle-treated OBX mice. Data was analyzed using Dunnett’s multiple comparison test. Veh, vehicle treatment. lent amounts of protein based on Bradford analysis were boiled 3 min in Laemmli sample buffer and subjected to SDS-polyacrylamide gel electrophoresis. Proteins were transferred to an Immobilon polyvinylidene difluoride membrane for 2 h at 70 V. After blocking with TTBS solution (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% Tween 20) containing 2.5% bovine serum albumin for 1 h at room temperature, membranes were incubated overnight at 4˚C with anti-ChAT, anti-phospho-Akt (Thr-308), anti-Akt, anti-phospho-ERK (Thr-202/Tyr-204) (1:2000; Sigma-Aldrich, St. Louis, MO), and anti-ERK (1:1000; Sigma-Aldrich). Bound antibodies were visualized using the enhanced chemiluminescence detection system (GE Healthcare, Chalfont St. Giles, UK) and analyzed semiquantitatively using the Image J program (National Institutes of Health, Bethesda, MD). Copyright © 2013 SciRes. OPEN ACCESS 164 Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 Figure 2. Effects of donepezil on phosphorylation of Akt and ERK in OBX mouse medial septum. Immunoblotting was carried out using antibodies recognizing phospho- or total protein. (A) Representative immunoblots of Akt-Ser-473 phosphorylation is indicated. Quantitative analysis of relative Akt-Ser-473 phosphorylation in indicated groups was performed by densitometry. Total amounts of Akt protein were unchanged following OBX. (B) Representative immunoblots of ERK phosphorylation is indicated. Quantitative analysis of relative ERK phosphorylation in indicated groups was performed by densitometry. Total amounts of ERK protein were unchanged following OBX. Data are expressed as percentage of values versus sham-operated animals (mean ± S.E.M.). * P < 0.05, **P < 0.01 versus shamoperated mice and # P < 0.05, ##P < 0.01 versus vehicle-treated mice. Data was analyzed using Dunnett’s multiple comparison tests. Veh, vehicle treatment. Donepezil administration stimulates Akt on ERK phosphorylation in the cholinergic neurons. (C) Confocal microscopy images of double staining in the medial septum for phospho-Akt (green), ChAT (red), and merged images are shown. Enlarged images indicate boxed inset. Scale bar, 20 μm. (D) Confocal microscopy images of double staining in the medial septum for phospho-ERK (green), ChAT (red), and merged images. Enlarged images indicate boxed area. Scale bar, 20 μm. Done, donepezil (1 mg/kg) treatment. 2.6. Statistical Analysis Data were represented as means ± S.E.M. For the result of novel object recognition test was analyzed using the unpaired Student’s t-test. Statistical significance for differences among groups was tested by one-way analysis of variance (ANOVA), followed by a Dunnett’s test for multigroup comparisons. P < 0.05 indicated statistically significant differences. Figure 3. Expression of nicotinic acetylcholine receptor and its functions in the medial septal cholinergic neurons (A) Expression of nAChR is found at cholinergic neruron in the medial septum. Double stained-immunohistochemistry in the medial septum for ChAT (green) and α-Bungarotoxin (red) (upper) is performed. Enlarged images in square boxes were shown in the lower panels. Scale bars, 50 μm in low magnification and 20 μm in high magnification images. (B) Representative band of immunoblots using antibodies recognizing phosphor- or total protein of Akt (left) and ERK (right) are indicated. Quantitative analyses of phospho-Akt and ERK levels were performed by densitometry of immunoblots. Data are expressed as percentage of values of sham-operated animals (mean ± S.E.M.) (n = 6). * P < 0.05, **P < 0.01 versus OBX-operated animals and ##P < 0.01 versus donepezil-treated OBX mice. Data was analyzed using Dunnett’s multiple comparison tests. Done, donepezil (1 mg/kg) treatment. Meca, mecamylamine (10 μM) treatment. 3. RESULTS 3.1. Effects of Chronic Donepezil Administration on Impaired Memory-Related Behaviors in OBX Mice As expected, chronic donepezil administration improved the spatial working memory and cognitive function in OBX mice in Y-maze task test and a novel object recognition task. OBX mice exhibited a significant decrease in alternation behaviors compared with shamoperated mice in the Y-maze task (Sham, 66.7% ± 1.9%; OBX, 46.1% ± 2.1%; Figure 4(A)). Donepezil (1 mg/kg) treatment alone did not alter spatial working memory of sham-operated mice (60.8% ± 1.9%; Figure 4(A)). OBXinduced alternation behavior deficit was dose-dependently improved by donepezil administration at dose of 1 and 3 mg/kg (0.3 mg/kg, 51.6% ± 0.7%; 1 mg/kg, 55.5% Copyright © 2013 SciRes. OPEN ACCESS Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 165 ± 2.7%; 3 mg/kg, 55.5% ± 1.7%; Figure 4) compared with vehicle-treated OBX mice. Similarly, in the novel object recognition task, none of the groups showed a preference for left or right objects in the trial session. In the test session, OBX mice showed significant impairment by failing to discriminate between familiar and novel objects (50.6% ± 3.6%; Figure 4(B)), while sham-operated groups spent more exploratory time for the novel object (61.1% ± 2.2%; Figure 4(B)). Donepezil (1 mg/kg) treatment alone to sham mice did not affect the discrimination index (60.2% ± 2.6%; Figure 4(B)) compared to vehicle-treated sham mice. When OBX mice were treated with donepezil (1 or 3 mg/kg, but not 0.3 mg/kg), a higher exploratory preference for the novel object was observed in a dose-dependent manner (0.3 mg/kg, 52.1% ± 1.7%; 1 mg/kg, 58.1% ± 4.3%; 3 mg/kg, 63.3% ± 3.0%; Figure 4(B)). 3.2. Effects of Donepezil Administration on Cholinergic Neurodegeneration in OBX Mouse Medial Septum Since OBX induces cholinergic neurodegeneration in the medial septum [7], we determined whether donepezil elicits the neuroprotective effects on OBX-induced cholinergic neurodegeneration in medial septum. Consistent with previous observations, ChAT-immunoreactive neurons was largely and significantly decreased in the medial septum of OBX mice (45% of that in Sham-operated animals) (Figures 1(A) and (B)). Notably, chronic administration of donepezil (1 and 3 mg/kg; 14 days p.o.) significantly inhibited reduction of ChAT-positive cholinergic neurons (Figures 1(A) and (B)). Donepezil administration alone (1 mg/kg) did not affect the number of ChAT positive neurons in sham-operated animals (94.9% ± 3.0%; Figures 1(A) and (B)). Consistent with immunohistochemical observations, reduced ChAT protein levels were dose-dependently restored by chronic donepezil administration in OBX mouse medial septum (OBX, 66.3% ± 5.3%; 0.3 mg/kg, 77.7% ± 11.1%; 1 mg/kg, 103.5% ± 14.7%; 3 mg/kg, 101.6% ± 18.3% of that in sham-operated animals; Figure 1(C)). The maximal effect of donepezil on ChAT protein levels was obtained by 1 mg/kg administration. ChAT protein levels did not differ significantly between groups of sham mice treated by donepezil alone (89.9% ± 7.6%; Figure 1(C)). 3.3. Effects of Donepezil on Phospho-Rylation of Akt and ERK in OBX Mouse Medial Septum Previous study reported that donepezil activates PI3K/Akt pathways in brain, thereby preventing lipopolysaccharide-induced neuroinflammation [28]. DoneFigure 4. Effects of chronic donepezil administration on impaired memory-related behaviors in OBX mice. (A) Effect of donepezil OBX-induced impairment of spontaneous alternation behavior, and number of arm entries in the Y-maze. Each bar represents the mean ± S.E.M. **P < 0.01 versus sham-operated animals; # P < 0.05, ##P < 0.01 versus OBX group; data was analyzed using Dunnett’s multiple comparison test. n = 5 - 6 in each group. (B) Novel object recognition behaviors were tested in sham, OBX and donepezil (0.3 - 3.0 mg/kg) treated groups. Differences in exploratory preference were assessed between groups either in the training or test session. Bars indicate means ± S.E.M. (n = 8). * P < 0.05 in the same treatment group and in novel group (right) versus familiar (left). Data was analyzed using Student’s t-test. Veh, vehicle treatment. pezil also promotes NGF-induced ERK phosphorylation coincident with NGF-stimulated neurite outgrowth in PC12 cells [23]. Therefore, we speculated activation of PI3K/Akt and ERK pathways are involved in neuroprotection of cholinergic neurons by donepezil. Therefore, we first examined changes in Akt and ERK phosphorylation in the extracts from the medial septum by immunoblotting analyses using phospho-specific antibodies against Akt and ERK (Figure 2). Consistent with our previous observations, OBX resulted in a significant reduction of Akt phosphorylation after OBX without changes in Akt protein levels (51.0 ± 9.6%; Figure 2(A)). Chronic donepezil administration significantly and completely restored the reduced Akt phosphorylation by the same levels of sham mice (0.3 mg/kg, 72.0% ± 12.9%; 1 mg/kg, 100.2% ± 11.7%; 3 mg/kg, 89.7% ± 11.8%; Figure 2(A)). Similarly, ERK phosphorylation in the medial septum of OBX was markedly reduced (63.4% ± 7.8%; Figure 2(B)) without changes in ERK protein levels. Donepezil administration also dose-dependently restored ERK phosphorylation comparable to levels seen in sham mice (0.3 mg/kg, 74.9% ± 2.4%; 1 mg/kg, 98.9% ± 14.7%; Copyright © 2013 SciRes. OPEN ACCESS 166 Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 3mg/kg, 92.5% ± 9.2%; Figure 2(B)). Taken together, donepezil chronic administration stimulated both Akt and ERK signaling pathways, thereby likely preventing OBX-induced cholinergic neurodegeneration in the medial septum. 3.4. Effects of Donepezil on Akt and ERK Phosphorylation in the Medial Septal Cholinergic Neurons We next confirmed that donepezil-induced enhancement of Akt and ERK phosphorylation occurs in the medial septal cholinergic neurons by immunohistochemical analyses. Interestingly, phosphorylated Akt immunoreactivity was predominantly localized in cell bodies of ChAT-positive cholinergic neurons in the medial septum with or without donepezil administration (1 mg/kg) in sham-operated mice (Figure 2(C)). In OBX mice, the number of phosphorylated Akt/ChAT double-positive neurons was remarkably decreased and the immunofluorescence against phosphorylated Akt relatively weak in ChAT-positive neurons. Chronic donepezil administration (1 mg/kg) restored the number of double-positive neurons and immunofluorescence against phosphorylated Akt in ChAT-positive neurons. Similarly, phosphorylated ERK (p-ERK) immunoreactivity was predominantly localized in cell bodies of ChAT-positive cholinergic neurons in the medial septum. Coincident with decrease in the number of ChATpositive neurons, phosphorylated ERK immunofluorescence was also decreased following OBX surgery and restored by donepezil administration in the medial septum as compared with sham-operated animals (Figure 2(D)). 3.5. Expression of Nicotinic Acetylcholine Receptor in the Medial Septal Cholinergic Neurons ChAT-positive cholinergic neurons of the medial septum is known to express α7 nAChR mRNA in rat [29]. Recent study also suggests that medial septal/diagonal band neurons express functional nAChRs [30]. To determine whether α7 nAChR is expressed in ChAT-positive cholinergic neuron in the medial septum, we performed double staining of the medial septum sections with anti-ChAT antibody and fluorescence-conjugated α-bungarotoxin (a selective ligand for α7 nAChR) in a normal mouse. α-bungarotoxin-labeled α7 nAChR was highly expressed in the most ChAT-positive cholinergic neurons in the medial septum (Figure 3(A)). However, other ChAT-negative cells were moderately stained with α-bungarotoxin, suggesting that other neurons or astrocytes may also express α7 nAChR. 3.6. Function of nAChR in DonepezilInduced Akt and ERK Activation in Medial Septum of OBX Mice We next evaluated whether donepezil-induced elevated phosphorylation of Akt and ERK is mediated by nAChR stimulation in the medial septum. Consistent with increased phosphorylation of Akt and ERK by chronic administration of donepezil, acute donepezil administration caused significant elevation of Akt and ERK phosphorylation compared with saline-treated OBX mice (Akt, 142.4% ± 7.2%; ERK, 132.2% ± 7.4%; Figure 3(B)). Importantly, mecamylamine pre-treatment completely abolished the elevated phosphorylation Akt and ERK (Akt, 63.97% ± 11.5%; ERK, 99.0% ± 7.4%; Figure 3(B)). These results suggest that donepezil-induced activation of Akt and ERK is mediated by nAChR stimulation. 3.7. Effects of Donepezil on CREB Phosphorylation and BDNF Expression in OBX Mouse Medial Septum We finally defined the physiological relevance of Akt and ERK activation by donepezil administration in the medial septum. We measured downstream signaling targets of Akt and ERK pathways. As previously reported [31], CREB phosphorylation levels in OBX mice significantly reduced compared with sham mice (59.4% ± 8.0%; Figure 5). Decreased level of CREB phosphorylation was restored by repeated donepezil treatment (1 mg/kg) (102.5% ± 8.8%; Figure 5). Unlike CREB phosphorylation, BDNF protein levels in OBX mice showed no significant changes by OBX treatment (118.0% ± 3.0%; Figure 5). However, BDNF protein level was significantly increased by donepezil treatment (141.3% ± 6.4%; Figure 5). Thus, donepezil-induced CREB phosphorylation may in part mediate the BDNF expression. 4. DISCUSSION Here, we for the first time demonstrated that an AChE inhibitor, donepezil, protects the septo-hippocampal cholinergic neurons against OBX-induced neurodegeneration. Previous studies reported that chronic donepezil treatment improved learning deficits in AD model mice and AD patients [12,32-34]. The septo-hippocampal cholinergic innervation was also impaired in the CA3 hippocampal regions following OBX [7], suggesting that the reduced septo-hippocampal cholinergic innervation accounts for impairment of learning and memory in OBX mice. Consistent with those observations, we confirmed that donepezil-induced neuroprotection of the cholinergic neurons is associated with improved cognitive behaviors in OBX mice. Other investigators have shown that Copyright © 2013 SciRes. OPEN ACCESS Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 167 Figure 5. Effects of donepezil on CREB phosphorylation and BDNF expression in OBX mouse medial septum. Phospho-CREB and BDNF levels were measured after OBX by immunoblotting with or without administration of donepezil (1 mg/kg). Quantitative analyses of phospho-CREB and BDNF levels were performed by densitometry of immunoblots. Data are expressed as percentage of values of sham-operated animals (mean ± S.E.M.) (n = 6). * P < 0.05, **P < 0.01 versus sham-operated animals and # P < 0.05 versus vehicletreated OBX mice. Data was analyzed using Dunnett’s multiple comparison tests. Done, donepezil (1 mg/kg) treatment. donepezil have neuroprotective effects against glutamate- or Aβ-induced neuronal cell death in cultured neurons [35,36]. We previously documented that Akt and ERK activities are decreased in the medial septum 14 days after OBX, with a concomitant reduce the number of ChATpositive neurons [7]. We here confirmed that decreased Akt and ERK occur in the ChAT-positive neurons in the medal septum. Donepezil prevents neurotoxicity in cultured cortical neurons through stimulation of Akt and ERK pathways via nAChRs [22,24,37,38]. We also defined that α7 nAChR is highly expressed in ChAT-positive cholinergic neurons in addition to ChAT-negative neurons in the medial septum as shown in Figure 3. Although the total protein levels of Akt and ERK were unchanged in the medial septum by OBX, the basal phosphorylated Akt and ERK immunoreactivities were predominantly observed in ChAT-positive neurons, suggesting that Akt and ERK activities are higher than those in ChAT-negative neurons in the medial septum. Furthermore, the enhancements of phosphorylated Akt and ERK immunoreactivity were seen predominantly in ChAT-positive neurons in the medial septum, suggesting that donepezil primary activates Akt and ERK the cholinergic neurons in the medial septum. Neuroprotective effect of donepezil was blocked by co-administration of PI3K/Akt inhibitor, LY294002, and mitogen-activated protein kinase kinase (MAPKK) inhibitor, PD98059 in rat cortical cultured neurons and/or SH-SY5Y neuroblastoma cells [17,38,39]. CREB phosphorylation is likely mediated by the increased ERK and/or PI3K/Akt signaling [40,41]. Furthermore, BDNF expression as downstream targets of Akt and/or ERK signaling mediates donepezil-induced neuroprotection in the medial septum. Indeed, BDNF level in the serum decreased in AD patients and it elevates after 15 months of treatment with donepezil [42]. We also demonstrated that α7 nAChR expressed in medial septal cholinergic neurons. Among nAChR, heteromeric α4β2- and homomeric α7-nAChRs are the most abundant isoforms in the mammalian brain [43]. Indeed, anti-α4nAChR immunoreactivity was observed in the medal septum of CBA/J mice [44]. Recently, novel heteromeric α7β2 nAChR is found in the rodent basal forebrain cholinergic neurons [45]. The α7β2 nAChR was major composition in the rat medial septum. Interestingly Aβ (1 - 42) strongly inhibits the α7β2 nAChR current in the cholinergic neurons isolated from the medial septum [45]. Taken together with our studies, α7-containing nAChR is major isoforms and function in the medial septum. Notably, a neuroprotective effect of nicotine and donepezil were mediated by activation of non-receptortype tyrosine kinase, Janus-activated kinase 2 (JAK2) and Fyn via α7 nAChR in rat cortical neurons [22]. This effect leads to phosphorylation of PI3K/Akt and ERK signaling, and facilitates transcription of target genes, including those involved in neuronal survival, such as bcl-2 and BDNF [46-48]. Other group also showed that acute donepezil administration in mice activates neurotrophin receptor, Trk receptors in the hippocampus [49], suggesting that neuroprotective effects of donepezil through nAChR induce BDNF elevation in the hippocampus. Thus nAChR likely accounts for the BDNF expression not only in the hippocampus but also in the meCopyright © 2013 SciRes. OPEN ACCESS 168 Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 dial septum, where the cholinergic neurons innervate the hippocampus. In conclusion, our findings indicate that cholinergic neurodegeneration in the septo-hippocampal system partly contributes to OBX-induced memory-related behavior deficits. Donepezil treatment to OBX mice significantly rescued cholinergic neurons in the medial septum through Akt/ERK survival signals activation, including CREB phosphorylation and BDNF expression. Donepezil-induced protection of septo-hippocampal cholinergic neurons is potential mechanism to prevent or delay cognitive impairments in AD. Further studies are required to define cells expressing CREB phosphorylation and BDNF in the medial septal neurons, because α7 nAChR is ubiquitously expressed in the medial septal neurons. 5. ACKNOWLEDGEMENTS This work was supported in part by a Grant-in-Aid for Scientific Research (B) from the Ministry of Education Culture, Sports, Science and Technology, Japan (22390109, K.F.) and the Smoking Research Foundation (K.F.). REFERENCES [1] Sieck, M.H. (1972) The role of the olfactory system in avoidance learning and activity. Physiology and Behavior, 8, 705-710. http://dx.doi.org/10.1016/0031-9384(72)90099-6 [2] Serby, M., Corwin, J., Conrad, P. and Rotrosen, J. (1985) Olfactory dysfunction in Alzheimer’s disease and Parkinson’s disease. The American Journal of Psychiatry, 142, 781-782. [3] Koss, E. (1986) Olfactory dysfunction in Alzheimer’s disease. Developmental Neuropsychology, 2, 89-99. http://dx.doi.org/10.1080/87565648609540332 [4] Esiri, M.M. and Wilcock, G.K. (1984) The olfactory bulbs in Alzheimer’s disease. Journal of Neurology, Neurosurgery and Psychiatry, 47, 56-60. http://dx.doi.org/10.1136/jnnp.47.1.56 [5] Doty, R.L. (1991) Olfactory capacities in aging and Alzheimer’s disease: Psychophysical and anatomic considerations. Annals of the New York Academy of Sciences, 640, 20-27. [6] Ferreira, G., Meurisse, M., Tillet, Y. and Lévy, F. (2001) Distribution and co-localization of choline acetyltransferase and p75 neurotrophin receptors in the sheep basal forebrain implications for the use of a specific cholinergic immunotoxin. Neuroscience, 104, 419-439. http://dx.doi.org/10.1016/S0306-4522(01)00075-6 [7] Han, F., Shioda, N., Moriguchi, S., Qin, Z.H. and Fukunaga, K. (2008) The vanadium (IV) compound rescues septo-hippocampal cholinergic neurons from neurodegeneration in olfactory bulbectomized mice. Neuroscience, 151, 671-679. http://dx.doi.org/10.1016/j.neuroscience.2007.11.011 [8] Nakajima, A., Yamakuni, T., Haraguchi, M., Omae, N., Song, S.Y., Kato, C., Nakagawasai, O., Tadano, T., Yokosuka, A. and Mimaki, Y. (2007) Nobiletin, a citrus flavonoid that improves memory impairment, rescues bulbectomy-induced cholinergic neurodegeneration in mice. Journal of Pharmacological Science, 105, 122-126. http://dx.doi.org/10.1254/jphs.SC0070155 [9] Han, F., Shioda, N., Moriguchi, S., Yamamoto, Y., Raie, A.Y., Yamaguchi, Y., Hino, M. and Fukunaga, K. (2008) Spiro[imidazo[1,2-a]pyridine-3,2-indan]-2(3H)-one (ZSET- 1446/ST101) treatment rescues olfactory bulbectomyinduced memory impairment by activating Ca2+/calmodulin kinase II and protein kinase C in mouse hippocampus. Journal of Pharmacology and Experimental Therapeutics, 326, 127-134. http://dx.doi.org/10.1124/jpet.108.137471 [10] Yamamoto, Y., Shioda, N., Han, F., Moriguchi, S. and Fukunaga, K. (2013) The novel cognitive enhancer ST101 enhances acetylcholine release in mouse dorsal hippocampus through T-type voltage-gated calcium channel stimulation. Journal of Pharmacological Sciences, 121, 212-226. http://dx.doi.org/10.1254/jphs.12233FP [11] Aleksandrova, I.Y., Kuvichkin, V.V., Kashparov, I.A., Medvinskaya, N.I., Nesterova, I.V., Lunin, S.M., Samokhin, A.N., Bobkova, N.V. (2004) Increased level of betaamyloid in the brain of bulbectomized mice. Biochemistry, 69, 176-180. [12] Winblad, B., Kilander, L., Eriksson, S., Minthon, L., Båtsman, S., Wetterholm, A.L., Jansson-Blixt, C. and Haglund, A. (2006) Donepezil in patients with severe Alzheimer’s disease: Double-blind, parallel-group, placebo-controlled study. Lancet, 367, 1057-1065. http://dx.doi.org/10.1016/S0140-6736(06)68350-5 [13] Seltzer, B. (2005) Donepezil: A review. Expert Opinion on Drug Metabolism and Toxicology, 1, 527-536. http://dx.doi.org/10.1517/17425255.1.3.527 [14] Naik, R.S., Hartmann, J., Kiewert, C., Duysen, E.G., Lockridge, O. and Klein, J. (2009) Effects of rivastigmine and donepezil on brain acetylcholine levels in acetylcholinesterase-deficient mice. Journal of Pharmacy and Pharmaceutical Science, 12, 79-85. [15] Takada, Y., Yonezawa, A., Kume, T., Katsuki, H., Kaneko, S., Sugimoto, H. and Akaike, A. (2003) Nicotinic acetylcholine receptor-mediated neuroprotection by donepezil against glutamate neurotoxicity in rat cortical neurons. Journal of Pharmacology and Experimental Therapeutics, 306, 772-777. http://dx.doi.org/10.1124/jpet.103.050104 [16] Akasofu, S., Kosasa, T., Kimura, M. and Kubota, A. (2003) Protective effect of donepezil in a primary culture of rat cortical neurons exposed to oxygen-glucose deprivation. European Journal of Pharmacology, 472, 57-63. http://dx.doi.org/10.1016/S0014-2999(03)01865-X [17] Shen, H., Kihara, T., Hongo, H., Wu, X., Kem, W.R., Shimohama, S., Akaike, A., Niidome, T. and Sugimoto, H. (2010) Neuroprotection by donepezil against glutamate excitotoxicity involves stimulation of alpha7 nicotinic receptors and internalization of NMDA receptors. British Journal of Pharmacology, 161, 127-139. http://dx.doi.org/10.1111/j.1476-5381.2010.00894.x Copyright © 2013 SciRes. OPEN ACCESS Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 169 [18] Yuan, H., Wang, W.P., Feng, N., Wang, L. and Wang, X.L. (2011) Donepezil attenuated oxygen-glucose deprivation insult by blocking Kv2.1 potassium channels. European Journal of Pharmacology, 657, 76-83. http://dx.doi.org/10.1016/j.ejphar.2011.01.054 [19] Saxena, G., Singh, S.P., Agrawal, R. and Nath, C. (2008) Effect of donepezil and tacrine on oxidative stress in intracerebral streptozotocin-induced model of dementia in mice. European Journal of Pharmacology, 581, 283-289. http://dx.doi.org/10.1016/j.ejphar.2007.12.009 [20] Min, D., Mao, X., Wu, K., Cao, Y., Guo, F., Zhu, S., Xie, N., Wang, L., Chen, T., Shaw, C. and Cai, J. (2012) Donepezil attenuates hippocampal neuronal damage and cognitive deficits after global cerebral ischemia in gerbils. Neuroscience Letters, 10, 29-33. http://dx.doi.org/10.1016/j.neulet.2011.12.064 [21] Hashimoto, M., Kazui, H., Matsumoto, K., Nakano, Y., Yasuda, M. and Mori, E. (2005) Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer’s disease? The American Journal of Psychiatry, 162, 676-682. http://dx.doi.org/10.1176/appi.ajp.162.4.676 [22] Takada-Takatori, Y., Kume, T., Sugimoto, M., Katsuki, H., Sugimoto, H. and Akaike, A. (2006) Acetylcholinesterase inhibitors used in treatment of Alzheimer’s disease prevent glutamate neurotoxicity via nicotinic acetylcholine receptors and phosphatidylinositol 3-kinase cascade. Neuropharmacology, 51, 474-486. http://dx.doi.org/10.1016/j.neuropharm.2006.04.007 [23] Oda, T., Kume, T., Katsuki, H., Niidome, T., Sugimoto, H. and Akaike, A. (2007) Donepezil potentiates nerve growth factor-induced neurite outgrowth in PC12 cells. Journal of Pharmacological Science, 104, 349-354. http://dx.doi.org/10.1254/jphs.FP0070563 [24] Noh, M.Y., Koh, S.H., Kim, Y., Kim, H.Y., Cho, G.W. and Kim, S.H. (2009) Neuroprotective effects of donepezil through inhibition of GSK-3 activity in amyloid-betainduced neuronal cell death. Journal of Neurochemistry, 108, 1116-1125. http://dx.doi.org/10.1111/j.1471-4159.2008.05837.x [25] Toborek, M., Son, K.W., Pudelko, A., King-Pospisil, K., Wylegala, E. and Malecki, A. (2007) ERK 1/2 signaling pathway is involved in nicotine-mediated neuroprotection in spinal cord neurons. Journal of Cellular Biochemistry, 100, 279-292. http://dx.doi.org/10.1002/jcb.21013 [26] Hozumi, S., Nakagawasai, O., Tan-No, K., Niijima, F., Yamadera, F., Murata, A., Arai, Y., Yasuhara, H. and Tadano, T. (2003) Characteristics of changes in cholinergic function and impairment of learning and memory-related behavior induced by olfactory bulbectomy. Behavioral Brain Research, 138, 9-15. http://dx.doi.org/10.1016/S0166-4328(02)00183-3 [27] Ennaceur, A. and Aggleton, J.P. (1997) The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behavioral Brain Research, 88, 181-193. http://dx.doi.org/10.1016/S0166-4328(97)02297-3 [28] Tyagi, E., Agrawal, R., Nath, C. and Shukla, R. (2010) Cholinergic protection via alpha7 nicotinic acetylcholine receptors and PI3K-Akt pathway in LPS-induced neuroinflammation. Neurochemistry International, 56, 135-142. http://dx.doi.org/10.1016/j.neuint.2009.09.011 [29] Azam, L., Winzer-Serhan, U. and Leslie, F.M. (2003) Coexpression of 7 and 2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience, 119, 965-977. http://dx.doi.org/10.1016/S0306-4522(03)00220-3 [30] Thinschmidt, J.S., Frazier, C.J., King, M.A., Meyer, E.M. and Papke, R.L. (2005) Medial septal/diagonal band cells express multiple functional nicotinic receptor subtypes that are correlated with firing frequency. Neuroscience Letters, 389, 163-168. http://dx.doi.org/10.1016/j.neulet.2005.07.038 [31] Moriguchi, S., Yamamoto, Y., Ikuno, T. and Fukunaga, K. (2011) Sigma-1 receptor stimulation by dehydroepiandrosterone ameliorates cognitive impairment through activation of CaM kinase II, protein kinase C and extracellular signal-regulated kinase in olfactory bulbectomized mice. Journal of Neurochemistry, 117, 879-891. http://dx.doi.org/10.1111/j.1471-4159.2011.07256.x [32] Ogura, H., Kosasa, T., Kuriya, Y. and Yamanishi, Y. (2000) Donepezil, a centrally acting acetylcholinesterase inhibitor, alleviates learning deficits in hypocholinergic models in rats. Methods and Findings in Experimental and Clinical Pharmacology, 22, 89-95. http://dx.doi.org/10.1358/mf.2000.22.2.796070 [33] Dong, H., Csernansky, C.A., Martin, M.V., Bertchume, A., Vallera, D. and Csernansky, J.G. (2005) Acetylcholinesterase inhibitors ameliorate behavioral deficits in the Tg2576 mouse model of Alzheimer’s disease. Psychopharmacology, 181, 145-152. http://dx.doi.org/10.1007/s00213-005-2230-6 [34] Van Dam, D., Abramowski, D., Staufenbiel, M. and De Deyn, P.P. (2005) Symptomatic effect of donepezil, rivastigmine, galantamine and memantine on cognitive deficits in the APP23 model. Psychopharmacology, 180, 177-190. http://dx.doi.org/10.1007/s00213-004-2132-z [35] Kimura, M., Akasofu, S., Ogura, H. and Sawada, K. (2005) Protective effect of donepezil against Abeta(1-40) neurotoxicity in rat septal neurons. Brain Research, 1047, 72-84. http://dx.doi.org/10.1016/j.brainres.2005.04.014 [36] Akasofu, S., Kimura, M., Kosasa, T., Sawada, K. and Ogura, H. (2008) Study of neuroprotection of donepezil, a therapy for Alzheimer’s disease. Chemico-Biological Interactions, 175, 222-226. http://dx.doi.org/10.1016/j.cbi.2008.04.045 [37] Akaike, A., Takada-Takatori, Y., Kume, T. and Izumi, Y. (2010) Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: Role of alpha4 and alpha7 receptors in neuroprotection. Journal of Molecular Neuroscience, 40, 211-226. http://dx.doi.org/10.1007/s12031-009-9236-1 [38] Takada-Takatori, Y., Kume, T., Ohgi, Y., Izumi, Y., Niidome, T., Fujii, T., Sugimoto, H. and Akaike, A. (2008) Mechanism of neuroprotection by donepezil pretreatment in rat cortical neurons chronically treated with donepezil. Journal of Neuroscience Research, 86, 3575-3583. http://dx.doi.org/10.1002/jnr.21798 Copyright © 2013 SciRes. OPEN ACCESS Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 Copyright © 2013 SciRes. OPEN ACCESS 170 [39] Arias, E., Gallego-Sandín, S., Villarroya, M., García, A.G. and López, M.G. (2005) Unequal neuroprotection afforded by the acetylcholinesterase inhibitors galantamine, donepezil, and rivastigmine in SH-SY5Y neuroblastoma cells role of nicotinic receptors. Journal of Pharmacology and Experimental Therapeutics, 315, 1346-1353. http://dx.doi.org/10.1124/jpet.105.090365 [40] Du, K. and Montminy, M. (1998) CREB is a regulatory target for the protein kinase Akt/PKB. Journal of Biological Chemistry, 273, 32377-32379. http://dx.doi.org/10.1074/jbc.273.49.32377 [41] Weeber, E.J. and Sweatt, J.D. (2002) Molecular neurobiology of human cognition. Neuron, 33, 845-848. http://dx.doi.org/10.1016/S0896-6273(02)00634-7 [42] Leyhe, T., Stransky, E., Eschweiler, G.W., Buchkremer, G. and Laske, C. (2008) Increase of BDNF serum concentration during donepezil treatment of patients with early Alzheimer’s disease. Journal of Alzheimer’s Disease, 16, 649-656. [43] Lindstrom, J. (1996) Neuronal nicotinic acetylcholine receptors. Ion Channels, 4, 377-450. [44] Roger, S.W., Gahring, L.C., Collins, A.C. and Marks, M. (1998) Age-related changes in neuronal nicotinic acetylcholine receptor subunit a4 expression are modified by long-term nicotine administration. The Journal of Neuroscience, 18, 4825-4832. [45] Liu, Q., Huang, Y., Xue, F., Simard, A., DeChon, J., Li, G., Zhang, J., Lucero, L., Wang, M., Sierks, M., Hu, G., Chang, Y., Lukas, R.J. and Wu, J. (2009) A novel nicotinic actylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. The Journal of Neuroscience, 29, 918-929. http://dx.doi.org/10.1523/JNEUROSCI.3952-08.2009 [46] Pugazhenthi, S., Nesterova, A., Sable, C., Heidenreich, K.A., Boxer, L.M., Heasley, L.E. and Reusch, J.E. (2000) Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. Journal of Biological Chemistry, 275, 10761-10766. http://dx.doi.org/10.1074/jbc.275.15.10761 [47] Hao, Y., Creson, T., Zhang, L., Li, P., Du, F., Yuan, P., Gould, T.D., Manji, H.K. and Chen, G. (2004) Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. Journal of Neuroscience, 24, 6590-6599. http://dx.doi.org/10.1523/JNEUROSCI.5747-03.2004 [48] Greer, P.L. and Greenberg, M.E. (2008) From synapse to nucleus: Calcium-dependent gene transcription in the control of synapse development and function. Neuron, 59, 846-860. http://dx.doi.org/10.1016/j.neuron.2008.09.002 [49] Autio, H., Mätlik, K., Rantamäki, T., Lindemann, L., Hoener, M.C., Chao, M., Arumäe, U. and Castrén, E. (2011) Acetylcholinesterase inhibitors rapidly activate Trk neurotrophin receptors in the mouse hippocampus. Neuropharmacology, 61, 1291-1296. http://dx.doi.org/10.1016/j.neuropharm.2011.07.033
Donepezil rescues the medical septum cholinergic neurons via nicotinic ACh receptor stimulation in olfactory bulbectomized mice Yui Yamamoto, Kohji Fukunaga* Department of Pharmacology, Graduate School of Pharmaceutical Science, Tohoku University, Sendai, Japan; * Corresponding Author: [email protected] Received 12 September 2013; revised 20 October 2013; accepted 1 November 2013 Copyright © 2013 Yui Yamamoto, Kohji Fukunaga. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Olfactory bulbectomy (OBX) causes cognitive dysfunction by degeneration of cholinergic neurons in the medial septum. Here, we define an involvement of nicotinic acetylcholine receptor (nAChR) in neuroprotective effect of donepezil in the septum neurons of OBX mice. Neuroprotective effects on the medial septal cholinergic neurons were assessed after chronic donepezil administration in OBX mice. We also measured Akt and ERK phosphorylation to define the neuroprotective mechanism of donepezil. We found that treatment with donepezil (1 - 3 mg/kg) for 15 consecutive days completely rescued cholinergic neurons in the OBX mice with concomitant improved memory. Reduction of both protein kinase B (Akt) and extracellular signal-regulated kinase (ERK) phosphorylation were restored by chronic donepezil administration (1 - 3 mg/kg) in OBX mouse medial septum. Both phosphorylated Akt and ERK immunoreactivities were localized in cell bodies of choline acetyltransferase (ChAT)-positive cholinergic cells in the medial septum. Enhancement of Akt and ERK phosphorylation seen following donepezil administration was totally blocked by pre-administration of mecamylamine (10 μM), a nicotinic acetylcholine receptor antagonist. Donepezil increases phosphorylation of Akt and ERK via nAChR stimulation in the medial septum cholinergic neurons. The Akt and ERK stimulation by donepezil is associated with its ability of neuroprotection in the medial septum and memory improvement. Keywords: Donepezil; Neuroprotection; Medial Septum; Nicotinic Acetylcholine Receptors 1. INTRODUCTION Destruction of olfactory system reportedly impairs learning and memory [1-3] and Alzheimer’s disease (AD) patients show severe impairment of olfactory bulb functions even in the early stage [4,5]. The cholinergic neurons in the basal forebrain and olfactory bulb are originated from the medial septum, thereby providing cholinergic innervation to the hippocampus, olfactory blub, amygdala and overall neocortex [6]. Interestingly, olfactory bulbectomy (OBX) causes degeneration of cholinergic neurons not only in the medial septum but also in the hippocampus, thereby eliciting hippocampus -dependent memory deficits in mice [7]. In support to this idea, OBX treatment elicits reduction of acetylcholinesterase (AChE) and choline acetyltransferase (ChAT) levels in the hippocampus [8,9] and nicotine-induced acetylcholine (ACh) release in the hippocampus was largely impaired in OBX mice [10]. In addition, significant elevation of betaamyloid (Aβ) levels is seen in extracts from neocortex and hippocampus by OBX in mice [11]. Thus, OBX mouse could be a useful model to test AD-like cognitive dysfunction following degeneration of cholinergic neurons in the septum. Donepezil, an AChE inhibitor, is prototyped therapeutics for Alzheimer’s disease (AD) patients in the worldwide [12]. Donepezil elevates extracellular ACh levels in the cortex [13,14]. Interestingly, neuroprotective effects of donepezil were also found both in vitro and in vivo. For example, donepezil blocked glutamate-induced excitotoxicity in rat primary cultured neurons and oxygen-glucose deprivation-induced cell death in HEK293 Copyright © 2013 SciRes. OPEN ACCESS 162 Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 cells and cortical neurons [15-18]. Donepezil also attenuates streptozotocin-induced or traumatic brain injury-induced neuronal death in hippocampal CA1 region, thereby improving cognitive impairment [19,20]. In human AD patients, donepezil slowed inhibition of hippocampal atrophy [21]. However, the mechanism underlying donepezil-mediated neuroprotection remains unclear. Donepezil treatment activated phosphatidylinositol 3- kinase (PI3K)/protein kinase B (Akt) and increased expression of Bcl-2, an anti-apoptotic protein, in rat cortical neurons [22]. Donepezil also potentiates the nerve growth factor (NGF)-induced phosphorylation of extracellular signal-regulated kinase (ERK) in PC12 cells [23]. Furthermore, neuroprotective effects of donepezil were abolished by mecamylamine, the non-selective nicotinic acetylcholine receptors (nAChRs) inhibitor, but not with scopolamine, the muscarinic AChRs (mAChRs) inhibitor, in rat cortical neurons [22]. Increased Akt phosphorylation following donepezil treatment in Aβ42-exposed cortical cultured neurons was blocked by mecamylamine pre-treatment [24]. Nicotine-induced activation of ERK was also blocked by mecamylamine in cultured spinal cord neurons [25]. Here, we found that chronic donepezil administration rescue degeneration of medial septum cholinergic neurons observed in OBX mice. Furthermore, we defined the expression of nAChR in the medial septum cholinergic neurons and possible neuroprotective mechanisms of donepezil in OBX mouse medial septum. 2. MATERIALS AND METHODS 2.1. Animals Adult male DDY mice 6 weeks old (Nippon SLC, Hamamatsu, Japan) housed in cages with free access to food and water at a constant temperature (23˚C ± 1˚C) and humidity (55% ± 5%) with a 12-h light/dark cycle (09:00 - 21:00 hours). All experimental procedures using animals were approved by the Committee on Animal Experiments at Tohoku University Graduate School of Pharmaceutical Sciences. 2.2. Establishment of Bilateral Olfactory Bulbectomy Mice and Drug Treatment OBX mice were prepared as described [9,10,26]. Briefly, mice anesthetized with sodium pentobarbital (50 mg/kg, i.p., Dainippon, Osaka, Japan) were placed in a stereotaxic instrument. After exposure of the skull, 1-mm diameter holes were drilled on either side of the olfactory bulbs, which were then removed by gentle aspiration by a suction pump. Care was taken not to damage the frontal cortex. Holes were filled with a hemostatic sponge to avoid bleeding and the skin was closed. Sham-operated mice were treated similarly but bulbs were left intact. Drug administrations were begun 3 days after recovery period. Donepezil (0.3, 1 or 3 mg/kg, dissolved in distilled water or vehicle alone) was administered orally daily for 15 consecutive days until mice were sacrificed, or one time intraperitoneally 30 min before sacrificed at 18 days after OBX surgery. Mecamylamine (10 μM) or saline were injected into the left brain lateral cerebroventricle 60min before acute donepezil administration. Following sacrificed by decapitation, medial septum was dissected out for further analyses. 2.3. Behavioral Analyses Spontaneous alternation behavior in a Y-maze served as a spatial reference memory task. The maze was made of black acryl plate. Each arm was 40 cm long, 8 cm high, 3 cm wide at the bottom and all three arms converged at an equal angle. Each mouse was placed at the end of a fixed arm and allowed to move freely through the maze during an 8-min session. The sequence of arm entries was recorded manually. An alternation was defined as entries into all three arms on consecutive choices. The maximum number of alternations was defined as the total number of arms entered minus two, and the percentage of alternation was calculated as (actual alternations/maximum alternations) × 100. The total number of arms entered during the session was also determined. The novel object recognition task used to evaluate recognition memory [27]. This task is based on the tendency of rodents to discriminate a familiar from a new object. Mice were inpidually habituated to an openfield box (35 × 25 × 35 cm) for 2 consecutive days. The experimenter scoring behaviors were blinded to the treatment. During the acquisition phases, two objects of the same material were placed symmetrically in the center of the chamber for 10 min. One hour after acquisition phase training, one object was replaced by novel object, and exploratory behavior was again analyzed for 5 min. After each session, objects were thoroughly cleaned with 75% ethanol to prevent odor recognition. Exploration of an object was defined as rearing on the object or sniffing it at a distance of less than 1 cm, touching it with the nose, or both. Successful recognition of a previously explored object was reflected by preferential exploration of the novel object. Discrimination of spatial novelty was assessed by comparing the difference between time of exploration of the novel (right) and familiar object (left) and the total time spent exploring both objects. 2.4. Immunohistochemical Studies Sham and OBX mice were treated with vehicle or donepezil (1 mg/kg) from 3 days to 17 days after surgery. Copyright © 2013 SciRes. OPEN ACCESS Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 163 At 18 days after surgery, mice anesthetized and perfusion-fixed with 4% paraformaldehyde. Briefly, after fixation, 50 μm thick coronal sections were cut using a vibrating microtome (Dosaka EM Co. Ltd., Kyoto, Japan). Sections were incubated as follows: for 30 min in 0.1 M PBS (pH 7.4) containing 0.1% Triton X-100; for 1 h in PBS with 3% BSA (blocking solution); overnight with various primary antibodies in blocking solution at 4˚C. For ChAT-immunostaining, sections were incubated with goat anti-ChAT polyclonal antibody (1:1000) (Millipore Bioscience Research Reagents, Temecula, CA). After washing, sections were incubated with biotinylated anti-goat IgG (1:5000) (Jackson Immuno Research Laboratories, West Grove, PA, USA) in TNB buffer for 1 h, followed by streptavidin-horseradish peroxidase (1:5000) (NEN Life Science Products, Boston, MA) labeled for 2 h. Sections were then stained with tetramethylrhodamine tyramide for 10 min using the TSA-Direct kit (NEN Life Science Products). For double staining with ChAT and phosphorylated Akt or ERK, sections were incubated with goat anti-ChAT polyclonal antibody (1:1000) (Millipore), a rabbit anti-phosphorylated Akt (Thr-308) polyclonal antibody (1:1000) (Millipore) or a rabbit antiphosphorylated ERK (Thr-202/Tyr-204) polyclonal antibody (1:1000) (Cell Signaling Technology, Beverly, MA, USA). After thorough washing, sections were incubated 3 h with Alexa 594-labeled anti-goat IgG or Alexa 488-labeled anti-rabbit IgG. To detect α7 nAChR, sections were incubated in α-bungarotoxin, tetramethylrhodamine conjugate (α-BTX) (Molecular Probes, Eugene, Oregon, USA) for 2 h at 37˚C, followed by incubation in antibodies for ChAT, phospho-Akt or phospho-ERK overnight at 4˚C. After several washes in PBS, sections were mounted on slides with Vectashield (Vector Laboratories, Burlingame, CA). Immunofluorescent images were analyzed using a confocal laser scanning microscope (Leica TCS, Olympus, Tokyo, Japan). 2.5. Immunoblotting Analysis In Figures 1 and 2, sham and OBX mice were treated with vehicle or donepezil (1 mg/kg) from 3 day to 17 days after OBX. And at 18 days, Medial septum was dissected out and stocked until homogenization. In Figure 3, sham and OBX mice were administrated donepezil (1 mg/kg) and mechamylamine as indicated. One hr after administration, the septum is dissected out from each mouse. The septum samples were homogenized in 70 µl of buffer containing 50 mM Tris-HCl, pH 7.4, 0.5% Triton X-100, 4 mM EGTA, 10 mM EDTA, 1 mM Na3VO4, 40 mM sodium pyrophosphate, 50 mM NaF, 100 nM calyculin A, 50 µg/ml leupeptin, 25 µg/ml pepstatin A, 50 µg/ml trypsin inhibitor, and 1 mM dithiothreitol. Insoluble material was removed by a 10 min centrifugation (15,000 rpm). Samples containing equivaFigure 1. Effects of donepezil administration on cholinergic neurodegeneration in OBX mouse medial septum. (A) Representative brain sections showing changes in numbers of cholinergic neurons. (a), cholinergic neurons were identified by ChAT immunostaining in sham (b), donepezil treated (1 mg/kg daily for 15 days) sham mice (c), vehicle (d), or donepezil treated (1, 3 mg/kg daily for 15 days) (e), (f) OBX mice. Confocal laser scanning images showed predominant ChAT expression in cell bodies of cholinergic neurons in the medial septum indicating in box (a). Scale bar, 150 μm. (B) Quantitative analysis of ChAT levels was undertaken by counting ChAT-positive neurons. Data are expressed as percentage of values of sham-operated animals (mean ± S.E.M.) (n = 6). * P < 0.05 versus sham-operated animals and # P < 0.05 versus vehicle-treated mice. Dunnett’s multiple comparison tests was used for data analysis. (C) ChAT levels were detected by immunoblotting with or without treatment with donepezil. Quantitative analysis of 68 KDa ChAT levels were performed by densitometric analysis of immunoblots. Immunoblots with β- tubulin showed equal protein loading. Data are expressed as percentage of values of sham-operated animals (mean ± S.E.M.) (n = 6). **P < 0.01 versus sham-operated animals and # P < 0.05 versus vehicle-treated OBX mice. Data was analyzed using Dunnett’s multiple comparison test. Veh, vehicle treatment. lent amounts of protein based on Bradford analysis were boiled 3 min in Laemmli sample buffer and subjected to SDS-polyacrylamide gel electrophoresis. Proteins were transferred to an Immobilon polyvinylidene difluoride membrane for 2 h at 70 V. After blocking with TTBS solution (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% Tween 20) containing 2.5% bovine serum albumin for 1 h at room temperature, membranes were incubated overnight at 4˚C with anti-ChAT, anti-phospho-Akt (Thr-308), anti-Akt, anti-phospho-ERK (Thr-202/Tyr-204) (1:2000; Sigma-Aldrich, St. Louis, MO), and anti-ERK (1:1000; Sigma-Aldrich). Bound antibodies were visualized using the enhanced chemiluminescence detection system (GE Healthcare, Chalfont St. Giles, UK) and analyzed semiquantitatively using the Image J program (National Institutes of Health, Bethesda, MD). Copyright © 2013 SciRes. OPEN ACCESS 164 Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 Figure 2. Effects of donepezil on phosphorylation of Akt and ERK in OBX mouse medial septum. Immunoblotting was carried out using antibodies recognizing phospho- or total protein. (A) Representative immunoblots of Akt-Ser-473 phosphorylation is indicated. Quantitative analysis of relative Akt-Ser-473 phosphorylation in indicated groups was performed by densitometry. Total amounts of Akt protein were unchanged following OBX. (B) Representative immunoblots of ERK phosphorylation is indicated. Quantitative analysis of relative ERK phosphorylation in indicated groups was performed by densitometry. Total amounts of ERK protein were unchanged following OBX. Data are expressed as percentage of values versus sham-operated animals (mean ± S.E.M.). * P < 0.05, **P < 0.01 versus shamoperated mice and # P < 0.05, ##P < 0.01 versus vehicle-treated mice. Data was analyzed using Dunnett’s multiple comparison tests. Veh, vehicle treatment. Donepezil administration stimulates Akt on ERK phosphorylation in the cholinergic neurons. (C) Confocal microscopy images of double staining in the medial septum for phospho-Akt (green), ChAT (red), and merged images are shown. Enlarged images indicate boxed inset. Scale bar, 20 μm. (D) Confocal microscopy images of double staining in the medial septum for phospho-ERK (green), ChAT (red), and merged images. Enlarged images indicate boxed area. Scale bar, 20 μm. Done, donepezil (1 mg/kg) treatment. 2.6. Statistical Analysis Data were represented as means ± S.E.M. For the result of novel object recognition test was analyzed using the unpaired Student’s t-test. Statistical significance for differences among groups was tested by one-way analysis of variance (ANOVA), followed by a Dunnett’s test for multigroup comparisons. P < 0.05 indicated statistically significant differences. Figure 3. Expression of nicotinic acetylcholine receptor and its functions in the medial septal cholinergic neurons (A) Expression of nAChR is found at cholinergic neruron in the medial septum. Double stained-immunohistochemistry in the medial septum for ChAT (green) and α-Bungarotoxin (red) (upper) is performed. Enlarged images in square boxes were shown in the lower panels. Scale bars, 50 μm in low magnification and 20 μm in high magnification images. (B) Representative band of immunoblots using antibodies recognizing phosphor- or total protein of Akt (left) and ERK (right) are indicated. Quantitative analyses of phospho-Akt and ERK levels were performed by densitometry of immunoblots. Data are expressed as percentage of values of sham-operated animals (mean ± S.E.M.) (n = 6). * P < 0.05, **P < 0.01 versus OBX-operated animals and ##P < 0.01 versus donepezil-treated OBX mice. Data was analyzed using Dunnett’s multiple comparison tests. Done, donepezil (1 mg/kg) treatment. Meca, mecamylamine (10 μM) treatment. 3. RESULTS 3.1. Effects of Chronic Donepezil Administration on Impaired Memory-Related Behaviors in OBX Mice As expected, chronic donepezil administration improved the spatial working memory and cognitive function in OBX mice in Y-maze task test and a novel object recognition task. OBX mice exhibited a significant decrease in alternation behaviors compared with shamoperated mice in the Y-maze task (Sham, 66.7% ± 1.9%; OBX, 46.1% ± 2.1%; Figure 4(A)). Donepezil (1 mg/kg) treatment alone did not alter spatial working memory of sham-operated mice (60.8% ± 1.9%; Figure 4(A)). OBXinduced alternation behavior deficit was dose-dependently improved by donepezil administration at dose of 1 and 3 mg/kg (0.3 mg/kg, 51.6% ± 0.7%; 1 mg/kg, 55.5% Copyright © 2013 SciRes. OPEN ACCESS Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 165 ± 2.7%; 3 mg/kg, 55.5% ± 1.7%; Figure 4) compared with vehicle-treated OBX mice. Similarly, in the novel object recognition task, none of the groups showed a preference for left or right objects in the trial session. In the test session, OBX mice showed significant impairment by failing to discriminate between familiar and novel objects (50.6% ± 3.6%; Figure 4(B)), while sham-operated groups spent more exploratory time for the novel object (61.1% ± 2.2%; Figure 4(B)). Donepezil (1 mg/kg) treatment alone to sham mice did not affect the discrimination index (60.2% ± 2.6%; Figure 4(B)) compared to vehicle-treated sham mice. When OBX mice were treated with donepezil (1 or 3 mg/kg, but not 0.3 mg/kg), a higher exploratory preference for the novel object was observed in a dose-dependent manner (0.3 mg/kg, 52.1% ± 1.7%; 1 mg/kg, 58.1% ± 4.3%; 3 mg/kg, 63.3% ± 3.0%; Figure 4(B)). 3.2. Effects of Donepezil Administration on Cholinergic Neurodegeneration in OBX Mouse Medial Septum Since OBX induces cholinergic neurodegeneration in the medial septum [7], we determined whether donepezil elicits the neuroprotective effects on OBX-induced cholinergic neurodegeneration in medial septum. Consistent with previous observations, ChAT-immunoreactive neurons was largely and significantly decreased in the medial septum of OBX mice (45% of that in Sham-operated animals) (Figures 1(A) and (B)). Notably, chronic administration of donepezil (1 and 3 mg/kg; 14 days p.o.) significantly inhibited reduction of ChAT-positive cholinergic neurons (Figures 1(A) and (B)). Donepezil administration alone (1 mg/kg) did not affect the number of ChAT positive neurons in sham-operated animals (94.9% ± 3.0%; Figures 1(A) and (B)). Consistent with immunohistochemical observations, reduced ChAT protein levels were dose-dependently restored by chronic donepezil administration in OBX mouse medial septum (OBX, 66.3% ± 5.3%; 0.3 mg/kg, 77.7% ± 11.1%; 1 mg/kg, 103.5% ± 14.7%; 3 mg/kg, 101.6% ± 18.3% of that in sham-operated animals; Figure 1(C)). The maximal effect of donepezil on ChAT protein levels was obtained by 1 mg/kg administration. ChAT protein levels did not differ significantly between groups of sham mice treated by donepezil alone (89.9% ± 7.6%; Figure 1(C)). 3.3. Effects of Donepezil on Phospho-Rylation of Akt and ERK in OBX Mouse Medial Septum Previous study reported that donepezil activates PI3K/Akt pathways in brain, thereby preventing lipopolysaccharide-induced neuroinflammation [28]. DoneFigure 4. Effects of chronic donepezil administration on impaired memory-related behaviors in OBX mice. (A) Effect of donepezil OBX-induced impairment of spontaneous alternation behavior, and number of arm entries in the Y-maze. Each bar represents the mean ± S.E.M. **P < 0.01 versus sham-operated animals; # P < 0.05, ##P < 0.01 versus OBX group; data was analyzed using Dunnett’s multiple comparison test. n = 5 - 6 in each group. (B) Novel object recognition behaviors were tested in sham, OBX and donepezil (0.3 - 3.0 mg/kg) treated groups. Differences in exploratory preference were assessed between groups either in the training or test session. Bars indicate means ± S.E.M. (n = 8). * P < 0.05 in the same treatment group and in novel group (right) versus familiar (left). Data was analyzed using Student’s t-test. Veh, vehicle treatment. pezil also promotes NGF-induced ERK phosphorylation coincident with NGF-stimulated neurite outgrowth in PC12 cells [23]. Therefore, we speculated activation of PI3K/Akt and ERK pathways are involved in neuroprotection of cholinergic neurons by donepezil. Therefore, we first examined changes in Akt and ERK phosphorylation in the extracts from the medial septum by immunoblotting analyses using phospho-specific antibodies against Akt and ERK (Figure 2). Consistent with our previous observations, OBX resulted in a significant reduction of Akt phosphorylation after OBX without changes in Akt protein levels (51.0 ± 9.6%; Figure 2(A)). Chronic donepezil administration significantly and completely restored the reduced Akt phosphorylation by the same levels of sham mice (0.3 mg/kg, 72.0% ± 12.9%; 1 mg/kg, 100.2% ± 11.7%; 3 mg/kg, 89.7% ± 11.8%; Figure 2(A)). Similarly, ERK phosphorylation in the medial septum of OBX was markedly reduced (63.4% ± 7.8%; Figure 2(B)) without changes in ERK protein levels. Donepezil administration also dose-dependently restored ERK phosphorylation comparable to levels seen in sham mice (0.3 mg/kg, 74.9% ± 2.4%; 1 mg/kg, 98.9% ± 14.7%; Copyright © 2013 SciRes. OPEN ACCESS 166 Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 3mg/kg, 92.5% ± 9.2%; Figure 2(B)). Taken together, donepezil chronic administration stimulated both Akt and ERK signaling pathways, thereby likely preventing OBX-induced cholinergic neurodegeneration in the medial septum. 3.4. Effects of Donepezil on Akt and ERK Phosphorylation in the Medial Septal Cholinergic Neurons We next confirmed that donepezil-induced enhancement of Akt and ERK phosphorylation occurs in the medial septal cholinergic neurons by immunohistochemical analyses. Interestingly, phosphorylated Akt immunoreactivity was predominantly localized in cell bodies of ChAT-positive cholinergic neurons in the medial septum with or without donepezil administration (1 mg/kg) in sham-operated mice (Figure 2(C)). In OBX mice, the number of phosphorylated Akt/ChAT double-positive neurons was remarkably decreased and the immunofluorescence against phosphorylated Akt relatively weak in ChAT-positive neurons. Chronic donepezil administration (1 mg/kg) restored the number of double-positive neurons and immunofluorescence against phosphorylated Akt in ChAT-positive neurons. Similarly, phosphorylated ERK (p-ERK) immunoreactivity was predominantly localized in cell bodies of ChAT-positive cholinergic neurons in the medial septum. Coincident with decrease in the number of ChATpositive neurons, phosphorylated ERK immunofluorescence was also decreased following OBX surgery and restored by donepezil administration in the medial septum as compared with sham-operated animals (Figure 2(D)). 3.5. Expression of Nicotinic Acetylcholine Receptor in the Medial Septal Cholinergic Neurons ChAT-positive cholinergic neurons of the medial septum is known to express α7 nAChR mRNA in rat [29]. Recent study also suggests that medial septal/diagonal band neurons express functional nAChRs [30]. To determine whether α7 nAChR is expressed in ChAT-positive cholinergic neuron in the medial septum, we performed double staining of the medial septum sections with anti-ChAT antibody and fluorescence-conjugated α-bungarotoxin (a selective ligand for α7 nAChR) in a normal mouse. α-bungarotoxin-labeled α7 nAChR was highly expressed in the most ChAT-positive cholinergic neurons in the medial septum (Figure 3(A)). However, other ChAT-negative cells were moderately stained with α-bungarotoxin, suggesting that other neurons or astrocytes may also express α7 nAChR. 3.6. Function of nAChR in DonepezilInduced Akt and ERK Activation in Medial Septum of OBX Mice We next evaluated whether donepezil-induced elevated phosphorylation of Akt and ERK is mediated by nAChR stimulation in the medial septum. Consistent with increased phosphorylation of Akt and ERK by chronic administration of donepezil, acute donepezil administration caused significant elevation of Akt and ERK phosphorylation compared with saline-treated OBX mice (Akt, 142.4% ± 7.2%; ERK, 132.2% ± 7.4%; Figure 3(B)). Importantly, mecamylamine pre-treatment completely abolished the elevated phosphorylation Akt and ERK (Akt, 63.97% ± 11.5%; ERK, 99.0% ± 7.4%; Figure 3(B)). These results suggest that donepezil-induced activation of Akt and ERK is mediated by nAChR stimulation. 3.7. Effects of Donepezil on CREB Phosphorylation and BDNF Expression in OBX Mouse Medial Septum We finally defined the physiological relevance of Akt and ERK activation by donepezil administration in the medial septum. We measured downstream signaling targets of Akt and ERK pathways. As previously reported [31], CREB phosphorylation levels in OBX mice significantly reduced compared with sham mice (59.4% ± 8.0%; Figure 5). Decreased level of CREB phosphorylation was restored by repeated donepezil treatment (1 mg/kg) (102.5% ± 8.8%; Figure 5). Unlike CREB phosphorylation, BDNF protein levels in OBX mice showed no significant changes by OBX treatment (118.0% ± 3.0%; Figure 5). However, BDNF protein level was significantly increased by donepezil treatment (141.3% ± 6.4%; Figure 5). Thus, donepezil-induced CREB phosphorylation may in part mediate the BDNF expression. 4. DISCUSSION Here, we for the first time demonstrated that an AChE inhibitor, donepezil, protects the septo-hippocampal cholinergic neurons against OBX-induced neurodegeneration. Previous studies reported that chronic donepezil treatment improved learning deficits in AD model mice and AD patients [12,32-34]. The septo-hippocampal cholinergic innervation was also impaired in the CA3 hippocampal regions following OBX [7], suggesting that the reduced septo-hippocampal cholinergic innervation accounts for impairment of learning and memory in OBX mice. Consistent with those observations, we confirmed that donepezil-induced neuroprotection of the cholinergic neurons is associated with improved cognitive behaviors in OBX mice. Other investigators have shown that Copyright © 2013 SciRes. OPEN ACCESS Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 167 Figure 5. Effects of donepezil on CREB phosphorylation and BDNF expression in OBX mouse medial septum. Phospho-CREB and BDNF levels were measured after OBX by immunoblotting with or without administration of donepezil (1 mg/kg). Quantitative analyses of phospho-CREB and BDNF levels were performed by densitometry of immunoblots. Data are expressed as percentage of values of sham-operated animals (mean ± S.E.M.) (n = 6). * P < 0.05, **P < 0.01 versus sham-operated animals and # P < 0.05 versus vehicletreated OBX mice. Data was analyzed using Dunnett’s multiple comparison tests. Done, donepezil (1 mg/kg) treatment. donepezil have neuroprotective effects against glutamate- or Aβ-induced neuronal cell death in cultured neurons [35,36]. We previously documented that Akt and ERK activities are decreased in the medial septum 14 days after OBX, with a concomitant reduce the number of ChATpositive neurons [7]. We here confirmed that decreased Akt and ERK occur in the ChAT-positive neurons in the medal septum. Donepezil prevents neurotoxicity in cultured cortical neurons through stimulation of Akt and ERK pathways via nAChRs [22,24,37,38]. We also defined that α7 nAChR is highly expressed in ChAT-positive cholinergic neurons in addition to ChAT-negative neurons in the medial septum as shown in Figure 3. Although the total protein levels of Akt and ERK were unchanged in the medial septum by OBX, the basal phosphorylated Akt and ERK immunoreactivities were predominantly observed in ChAT-positive neurons, suggesting that Akt and ERK activities are higher than those in ChAT-negative neurons in the medial septum. Furthermore, the enhancements of phosphorylated Akt and ERK immunoreactivity were seen predominantly in ChAT-positive neurons in the medial septum, suggesting that donepezil primary activates Akt and ERK the cholinergic neurons in the medial septum. Neuroprotective effect of donepezil was blocked by co-administration of PI3K/Akt inhibitor, LY294002, and mitogen-activated protein kinase kinase (MAPKK) inhibitor, PD98059 in rat cortical cultured neurons and/or SH-SY5Y neuroblastoma cells [17,38,39]. CREB phosphorylation is likely mediated by the increased ERK and/or PI3K/Akt signaling [40,41]. Furthermore, BDNF expression as downstream targets of Akt and/or ERK signaling mediates donepezil-induced neuroprotection in the medial septum. Indeed, BDNF level in the serum decreased in AD patients and it elevates after 15 months of treatment with donepezil [42]. We also demonstrated that α7 nAChR expressed in medial septal cholinergic neurons. Among nAChR, heteromeric α4β2- and homomeric α7-nAChRs are the most abundant isoforms in the mammalian brain [43]. Indeed, anti-α4nAChR immunoreactivity was observed in the medal septum of CBA/J mice [44]. Recently, novel heteromeric α7β2 nAChR is found in the rodent basal forebrain cholinergic neurons [45]. The α7β2 nAChR was major composition in the rat medial septum. Interestingly Aβ (1 - 42) strongly inhibits the α7β2 nAChR current in the cholinergic neurons isolated from the medial septum [45]. Taken together with our studies, α7-containing nAChR is major isoforms and function in the medial septum. Notably, a neuroprotective effect of nicotine and donepezil were mediated by activation of non-receptortype tyrosine kinase, Janus-activated kinase 2 (JAK2) and Fyn via α7 nAChR in rat cortical neurons [22]. This effect leads to phosphorylation of PI3K/Akt and ERK signaling, and facilitates transcription of target genes, including those involved in neuronal survival, such as bcl-2 and BDNF [46-48]. Other group also showed that acute donepezil administration in mice activates neurotrophin receptor, Trk receptors in the hippocampus [49], suggesting that neuroprotective effects of donepezil through nAChR induce BDNF elevation in the hippocampus. Thus nAChR likely accounts for the BDNF expression not only in the hippocampus but also in the meCopyright © 2013 SciRes. OPEN ACCESS 168 Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 dial septum, where the cholinergic neurons innervate the hippocampus. In conclusion, our findings indicate that cholinergic neurodegeneration in the septo-hippocampal system partly contributes to OBX-induced memory-related behavior deficits. Donepezil treatment to OBX mice significantly rescued cholinergic neurons in the medial septum through Akt/ERK survival signals activation, including CREB phosphorylation and BDNF expression. Donepezil-induced protection of septo-hippocampal cholinergic neurons is potential mechanism to prevent or delay cognitive impairments in AD. Further studies are required to define cells expressing CREB phosphorylation and BDNF in the medial septal neurons, because α7 nAChR is ubiquitously expressed in the medial septal neurons. 5. ACKNOWLEDGEMENTS This work was supported in part by a Grant-in-Aid for Scientific Research (B) from the Ministry of Education Culture, Sports, Science and Technology, Japan (22390109, K.F.) and the Smoking Research Foundation (K.F.). REFERENCES [1] Sieck, M.H. (1972) The role of the olfactory system in avoidance learning and activity. Physiology and Behavior, 8, 705-710. http://dx.doi.org/10.1016/0031-9384(72)90099-6 [2] Serby, M., Corwin, J., Conrad, P. and Rotrosen, J. (1985) Olfactory dysfunction in Alzheimer’s disease and Parkinson’s disease. The American Journal of Psychiatry, 142, 781-782. [3] Koss, E. (1986) Olfactory dysfunction in Alzheimer’s disease. Developmental Neuropsychology, 2, 89-99. http://dx.doi.org/10.1080/87565648609540332 [4] Esiri, M.M. and Wilcock, G.K. (1984) The olfactory bulbs in Alzheimer’s disease. Journal of Neurology, Neurosurgery and Psychiatry, 47, 56-60. http://dx.doi.org/10.1136/jnnp.47.1.56 [5] Doty, R.L. (1991) Olfactory capacities in aging and Alzheimer’s disease: Psychophysical and anatomic considerations. Annals of the New York Academy of Sciences, 640, 20-27. [6] Ferreira, G., Meurisse, M., Tillet, Y. and Lévy, F. (2001) Distribution and co-localization of choline acetyltransferase and p75 neurotrophin receptors in the sheep basal forebrain implications for the use of a specific cholinergic immunotoxin. Neuroscience, 104, 419-439. http://dx.doi.org/10.1016/S0306-4522(01)00075-6 [7] Han, F., Shioda, N., Moriguchi, S., Qin, Z.H. and Fukunaga, K. (2008) The vanadium (IV) compound rescues septo-hippocampal cholinergic neurons from neurodegeneration in olfactory bulbectomized mice. Neuroscience, 151, 671-679. http://dx.doi.org/10.1016/j.neuroscience.2007.11.011 [8] Nakajima, A., Yamakuni, T., Haraguchi, M., Omae, N., Song, S.Y., Kato, C., Nakagawasai, O., Tadano, T., Yokosuka, A. and Mimaki, Y. (2007) Nobiletin, a citrus flavonoid that improves memory impairment, rescues bulbectomy-induced cholinergic neurodegeneration in mice. Journal of Pharmacological Science, 105, 122-126. http://dx.doi.org/10.1254/jphs.SC0070155 [9] Han, F., Shioda, N., Moriguchi, S., Yamamoto, Y., Raie, A.Y., Yamaguchi, Y., Hino, M. and Fukunaga, K. (2008) Spiro[imidazo[1,2-a]pyridine-3,2-indan]-2(3H)-one (ZSET- 1446/ST101) treatment rescues olfactory bulbectomyinduced memory impairment by activating Ca2+/calmodulin kinase II and protein kinase C in mouse hippocampus. Journal of Pharmacology and Experimental Therapeutics, 326, 127-134. http://dx.doi.org/10.1124/jpet.108.137471 [10] Yamamoto, Y., Shioda, N., Han, F., Moriguchi, S. and Fukunaga, K. (2013) The novel cognitive enhancer ST101 enhances acetylcholine release in mouse dorsal hippocampus through T-type voltage-gated calcium channel stimulation. Journal of Pharmacological Sciences, 121, 212-226. http://dx.doi.org/10.1254/jphs.12233FP [11] Aleksandrova, I.Y., Kuvichkin, V.V., Kashparov, I.A., Medvinskaya, N.I., Nesterova, I.V., Lunin, S.M., Samokhin, A.N., Bobkova, N.V. (2004) Increased level of betaamyloid in the brain of bulbectomized mice. Biochemistry, 69, 176-180. [12] Winblad, B., Kilander, L., Eriksson, S., Minthon, L., Båtsman, S., Wetterholm, A.L., Jansson-Blixt, C. and Haglund, A. (2006) Donepezil in patients with severe Alzheimer’s disease: Double-blind, parallel-group, placebo-controlled study. Lancet, 367, 1057-1065. http://dx.doi.org/10.1016/S0140-6736(06)68350-5 [13] Seltzer, B. (2005) Donepezil: A review. Expert Opinion on Drug Metabolism and Toxicology, 1, 527-536. http://dx.doi.org/10.1517/17425255.1.3.527 [14] Naik, R.S., Hartmann, J., Kiewert, C., Duysen, E.G., Lockridge, O. and Klein, J. (2009) Effects of rivastigmine and donepezil on brain acetylcholine levels in acetylcholinesterase-deficient mice. Journal of Pharmacy and Pharmaceutical Science, 12, 79-85. [15] Takada, Y., Yonezawa, A., Kume, T., Katsuki, H., Kaneko, S., Sugimoto, H. and Akaike, A. (2003) Nicotinic acetylcholine receptor-mediated neuroprotection by donepezil against glutamate neurotoxicity in rat cortical neurons. Journal of Pharmacology and Experimental Therapeutics, 306, 772-777. http://dx.doi.org/10.1124/jpet.103.050104 [16] Akasofu, S., Kosasa, T., Kimura, M. and Kubota, A. (2003) Protective effect of donepezil in a primary culture of rat cortical neurons exposed to oxygen-glucose deprivation. European Journal of Pharmacology, 472, 57-63. http://dx.doi.org/10.1016/S0014-2999(03)01865-X [17] Shen, H., Kihara, T., Hongo, H., Wu, X., Kem, W.R., Shimohama, S., Akaike, A., Niidome, T. and Sugimoto, H. (2010) Neuroprotection by donepezil against glutamate excitotoxicity involves stimulation of alpha7 nicotinic receptors and internalization of NMDA receptors. British Journal of Pharmacology, 161, 127-139. http://dx.doi.org/10.1111/j.1476-5381.2010.00894.x Copyright © 2013 SciRes. OPEN ACCESS Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 169 [18] Yuan, H., Wang, W.P., Feng, N., Wang, L. and Wang, X.L. (2011) Donepezil attenuated oxygen-glucose deprivation insult by blocking Kv2.1 potassium channels. European Journal of Pharmacology, 657, 76-83. http://dx.doi.org/10.1016/j.ejphar.2011.01.054 [19] Saxena, G., Singh, S.P., Agrawal, R. and Nath, C. (2008) Effect of donepezil and tacrine on oxidative stress in intracerebral streptozotocin-induced model of dementia in mice. European Journal of Pharmacology, 581, 283-289. http://dx.doi.org/10.1016/j.ejphar.2007.12.009 [20] Min, D., Mao, X., Wu, K., Cao, Y., Guo, F., Zhu, S., Xie, N., Wang, L., Chen, T., Shaw, C. and Cai, J. (2012) Donepezil attenuates hippocampal neuronal damage and cognitive deficits after global cerebral ischemia in gerbils. Neuroscience Letters, 10, 29-33. http://dx.doi.org/10.1016/j.neulet.2011.12.064 [21] Hashimoto, M., Kazui, H., Matsumoto, K., Nakano, Y., Yasuda, M. and Mori, E. (2005) Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer’s disease? The American Journal of Psychiatry, 162, 676-682. http://dx.doi.org/10.1176/appi.ajp.162.4.676 [22] Takada-Takatori, Y., Kume, T., Sugimoto, M., Katsuki, H., Sugimoto, H. and Akaike, A. (2006) Acetylcholinesterase inhibitors used in treatment of Alzheimer’s disease prevent glutamate neurotoxicity via nicotinic acetylcholine receptors and phosphatidylinositol 3-kinase cascade. Neuropharmacology, 51, 474-486. http://dx.doi.org/10.1016/j.neuropharm.2006.04.007 [23] Oda, T., Kume, T., Katsuki, H., Niidome, T., Sugimoto, H. and Akaike, A. (2007) Donepezil potentiates nerve growth factor-induced neurite outgrowth in PC12 cells. Journal of Pharmacological Science, 104, 349-354. http://dx.doi.org/10.1254/jphs.FP0070563 [24] Noh, M.Y., Koh, S.H., Kim, Y., Kim, H.Y., Cho, G.W. and Kim, S.H. (2009) Neuroprotective effects of donepezil through inhibition of GSK-3 activity in amyloid-betainduced neuronal cell death. Journal of Neurochemistry, 108, 1116-1125. http://dx.doi.org/10.1111/j.1471-4159.2008.05837.x [25] Toborek, M., Son, K.W., Pudelko, A., King-Pospisil, K., Wylegala, E. and Malecki, A. (2007) ERK 1/2 signaling pathway is involved in nicotine-mediated neuroprotection in spinal cord neurons. Journal of Cellular Biochemistry, 100, 279-292. http://dx.doi.org/10.1002/jcb.21013 [26] Hozumi, S., Nakagawasai, O., Tan-No, K., Niijima, F., Yamadera, F., Murata, A., Arai, Y., Yasuhara, H. and Tadano, T. (2003) Characteristics of changes in cholinergic function and impairment of learning and memory-related behavior induced by olfactory bulbectomy. Behavioral Brain Research, 138, 9-15. http://dx.doi.org/10.1016/S0166-4328(02)00183-3 [27] Ennaceur, A. and Aggleton, J.P. (1997) The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behavioral Brain Research, 88, 181-193. http://dx.doi.org/10.1016/S0166-4328(97)02297-3 [28] Tyagi, E., Agrawal, R., Nath, C. and Shukla, R. (2010) Cholinergic protection via alpha7 nicotinic acetylcholine receptors and PI3K-Akt pathway in LPS-induced neuroinflammation. Neurochemistry International, 56, 135-142. http://dx.doi.org/10.1016/j.neuint.2009.09.011 [29] Azam, L., Winzer-Serhan, U. and Leslie, F.M. (2003) Coexpression of 7 and 2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience, 119, 965-977. http://dx.doi.org/10.1016/S0306-4522(03)00220-3 [30] Thinschmidt, J.S., Frazier, C.J., King, M.A., Meyer, E.M. and Papke, R.L. (2005) Medial septal/diagonal band cells express multiple functional nicotinic receptor subtypes that are correlated with firing frequency. Neuroscience Letters, 389, 163-168. http://dx.doi.org/10.1016/j.neulet.2005.07.038 [31] Moriguchi, S., Yamamoto, Y., Ikuno, T. and Fukunaga, K. (2011) Sigma-1 receptor stimulation by dehydroepiandrosterone ameliorates cognitive impairment through activation of CaM kinase II, protein kinase C and extracellular signal-regulated kinase in olfactory bulbectomized mice. Journal of Neurochemistry, 117, 879-891. http://dx.doi.org/10.1111/j.1471-4159.2011.07256.x [32] Ogura, H., Kosasa, T., Kuriya, Y. and Yamanishi, Y. (2000) Donepezil, a centrally acting acetylcholinesterase inhibitor, alleviates learning deficits in hypocholinergic models in rats. Methods and Findings in Experimental and Clinical Pharmacology, 22, 89-95. http://dx.doi.org/10.1358/mf.2000.22.2.796070 [33] Dong, H., Csernansky, C.A., Martin, M.V., Bertchume, A., Vallera, D. and Csernansky, J.G. (2005) Acetylcholinesterase inhibitors ameliorate behavioral deficits in the Tg2576 mouse model of Alzheimer’s disease. Psychopharmacology, 181, 145-152. http://dx.doi.org/10.1007/s00213-005-2230-6 [34] Van Dam, D., Abramowski, D., Staufenbiel, M. and De Deyn, P.P. (2005) Symptomatic effect of donepezil, rivastigmine, galantamine and memantine on cognitive deficits in the APP23 model. Psychopharmacology, 180, 177-190. http://dx.doi.org/10.1007/s00213-004-2132-z [35] Kimura, M., Akasofu, S., Ogura, H. and Sawada, K. (2005) Protective effect of donepezil against Abeta(1-40) neurotoxicity in rat septal neurons. Brain Research, 1047, 72-84. http://dx.doi.org/10.1016/j.brainres.2005.04.014 [36] Akasofu, S., Kimura, M., Kosasa, T., Sawada, K. and Ogura, H. (2008) Study of neuroprotection of donepezil, a therapy for Alzheimer’s disease. Chemico-Biological Interactions, 175, 222-226. http://dx.doi.org/10.1016/j.cbi.2008.04.045 [37] Akaike, A., Takada-Takatori, Y., Kume, T. and Izumi, Y. (2010) Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: Role of alpha4 and alpha7 receptors in neuroprotection. Journal of Molecular Neuroscience, 40, 211-226. http://dx.doi.org/10.1007/s12031-009-9236-1 [38] Takada-Takatori, Y., Kume, T., Ohgi, Y., Izumi, Y., Niidome, T., Fujii, T., Sugimoto, H. and Akaike, A. (2008) Mechanism of neuroprotection by donepezil pretreatment in rat cortical neurons chronically treated with donepezil. Journal of Neuroscience Research, 86, 3575-3583. http://dx.doi.org/10.1002/jnr.21798 Copyright © 2013 SciRes. OPEN ACCESS Y. Yamamoto, K. Fukunaga / Advances in Alzheimer’s Disease 2 (2013) 161-170 Copyright © 2013 SciRes. OPEN ACCESS 170 [39] Arias, E., Gallego-Sandín, S., Villarroya, M., García, A.G. and López, M.G. (2005) Unequal neuroprotection afforded by the acetylcholinesterase inhibitors galantamine, donepezil, and rivastigmine in SH-SY5Y neuroblastoma cells role of nicotinic receptors. Journal of Pharmacology and Experimental Therapeutics, 315, 1346-1353. http://dx.doi.org/10.1124/jpet.105.090365 [40] Du, K. and Montminy, M. (1998) CREB is a regulatory target for the protein kinase Akt/PKB. Journal of Biological Chemistry, 273, 32377-32379. http://dx.doi.org/10.1074/jbc.273.49.32377 [41] Weeber, E.J. and Sweatt, J.D. (2002) Molecular neurobiology of human cognition. Neuron, 33, 845-848. http://dx.doi.org/10.1016/S0896-6273(02)00634-7 [42] Leyhe, T., Stransky, E., Eschweiler, G.W., Buchkremer, G. and Laske, C. (2008) Increase of BDNF serum concentration during donepezil treatment of patients with early Alzheimer’s disease. Journal of Alzheimer’s Disease, 16, 649-656. [43] Lindstrom, J. (1996) Neuronal nicotinic acetylcholine receptors. Ion Channels, 4, 377-450. [44] Roger, S.W., Gahring, L.C., Collins, A.C. and Marks, M. (1998) Age-related changes in neuronal nicotinic acetylcholine receptor subunit a4 expression are modified by long-term nicotine administration. The Journal of Neuroscience, 18, 4825-4832. [45] Liu, Q., Huang, Y., Xue, F., Simard, A., DeChon, J., Li, G., Zhang, J., Lucero, L., Wang, M., Sierks, M., Hu, G., Chang, Y., Lukas, R.J. and Wu, J. (2009) A novel nicotinic actylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. The Journal of Neuroscience, 29, 918-929. http://dx.doi.org/10.1523/JNEUROSCI.3952-08.2009 [46] Pugazhenthi, S., Nesterova, A., Sable, C., Heidenreich, K.A., Boxer, L.M., Heasley, L.E. and Reusch, J.E. (2000) Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. Journal of Biological Chemistry, 275, 10761-10766. http://dx.doi.org/10.1074/jbc.275.15.10761 [47] Hao, Y., Creson, T., Zhang, L., Li, P., Du, F., Yuan, P., Gould, T.D., Manji, H.K. and Chen, G. (2004) Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. Journal of Neuroscience, 24, 6590-6599. http://dx.doi.org/10.1523/JNEUROSCI.5747-03.2004 [48] Greer, P.L. and Greenberg, M.E. (2008) From synapse to nucleus: Calcium-dependent gene transcription in the control of synapse development and function. Neuron, 59, 846-860. http://dx.doi.org/10.1016/j.neuron.2008.09.002 [49] Autio, H., Mätlik, K., Rantamäki, T., Lindemann, L., Hoener, M.C., Chao, M., Arumäe, U. and Castrén, E. (2011) Acetylcholinesterase inhibitors rapidly activate Trk neurotrophin receptors in the mouse hippocampus. Neuropharmacology, 61, 1291-1296. http://dx.doi.org/10.1016/j.neuropharm.2011.07.033


